Motoric response inhibition in finger movement and saccadic eye ...
Motoric response inhibition in finger movement and saccadic eye ...
Motoric response inhibition in finger movement and saccadic eye ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1060<br />
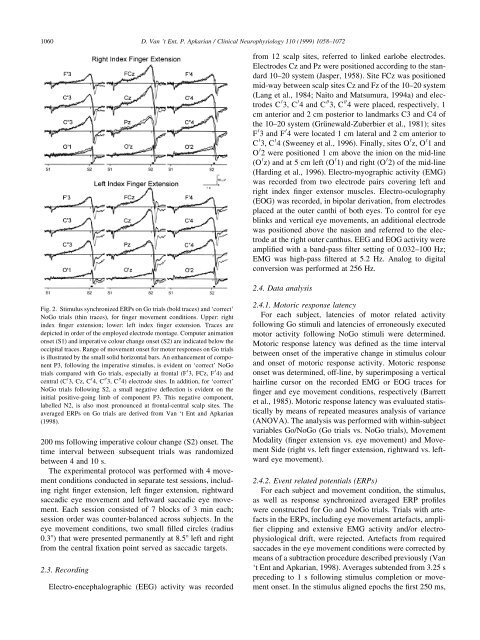
Fig. 2. Stimulus synchronized ERPs on Go trials (bold traces) <strong>and</strong> `correct'<br />
NoGo trials (th<strong>in</strong> traces), for ®nger <strong>movement</strong> conditions. Upper: right<br />
<strong>in</strong>dex ®nger extension; lower: left <strong>in</strong>dex ®nger extension. Traces are<br />
depicted <strong>in</strong> order of the employed electrode montage. Computer animation<br />
onset (S1) <strong>and</strong> imperative colour change onset (S2) are <strong>in</strong>dicated below the<br />
occipital traces. Range of <strong>movement</strong> onset for motor <strong>response</strong>s on Go trials<br />
is illustrated by the small solid horizontal bars. An enhancement of component<br />
P3, follow<strong>in</strong>g the imperative stimulus, is evident on `correct' NoGo<br />
trials compared with Go trials, especially at frontal (F 0 3, FCz, F 0 4) <strong>and</strong><br />
central (C 0 3, Cz, C 0 4, C 00 3, C 00 4) electrode sites. In addition, for `correct'<br />
NoGo trials follow<strong>in</strong>g S2, a small negative de¯ection is evident on the<br />
<strong>in</strong>itial positive-go<strong>in</strong>g limb of component P3. This negative component,<br />
labelled N2, is also most pronounced at frontal-central scalp sites. The<br />
averaged ERPs on Go trials are derived from Van `t Ent <strong>and</strong> Apkarian<br />
(1998).<br />
200 ms follow<strong>in</strong>g imperative colour change (S2) onset. The<br />
time <strong>in</strong>terval between subsequent trials was r<strong>and</strong>omized<br />
between 4 <strong>and</strong> 10 s.<br />
The experimental protocol was performed with 4 <strong>movement</strong><br />
conditions conducted <strong>in</strong> separate test sessions, <strong>in</strong>clud<strong>in</strong>g<br />
right ®nger extension, left ®nger extension, rightward<br />
<strong>saccadic</strong> <strong>eye</strong> <strong>movement</strong> <strong>and</strong> leftward <strong>saccadic</strong> <strong>eye</strong> <strong>movement</strong>.<br />
Each session consisted of 7 blocks of 3 m<strong>in</strong> each;<br />
session order was counter-balanced across subjects. In the<br />
<strong>eye</strong> <strong>movement</strong> conditions, two small ®lled circles (radius<br />
0.38) that were presented permanently at 8.58 left <strong>and</strong> right<br />
from the central ®xation po<strong>in</strong>t served as <strong>saccadic</strong> targets.<br />
2.3. Record<strong>in</strong>g<br />
Electro-encephalographic (EEG) activity was recorded<br />
D. Van 't Ent, P. Apkarian / Cl<strong>in</strong>ical Neurophysiology 110 (1999) 1058±1072<br />
from 12 scalp sites, referred to l<strong>in</strong>ked earlobe electrodes.<br />
Electrodes Cz <strong>and</strong> Pz were positioned accord<strong>in</strong>g to the st<strong>and</strong>ard<br />
10±20 system (Jasper, 1958). Site FCz was positioned<br />
mid-way between scalp sites Cz <strong>and</strong> Fz of the 10±20 system<br />
(Lang et al., 1984; Naito <strong>and</strong> Matsumura, 1994a) <strong>and</strong> electrodes<br />
C 0 3, C 0 4 <strong>and</strong> C 00 3, C 00 4 were placed, respectively, 1<br />
cm anterior <strong>and</strong> 2 cm posterior to l<strong>and</strong>marks C3 <strong>and</strong> C4 of<br />
the 10±20 system (GruÈnewald-Zuberbier et al., 1981); sites<br />
F 0 3 <strong>and</strong> F 0 4 were located 1 cm lateral <strong>and</strong> 2 cm anterior to<br />
C 0 3, C 0 4 (Sweeney et al., 1996). F<strong>in</strong>ally, sites O 0 z, O 0 1 <strong>and</strong><br />
O 0 2 were positioned 1 cm above the <strong>in</strong>ion on the mid-l<strong>in</strong>e<br />
(O 0 z) <strong>and</strong> at 5 cm left (O 0 1) <strong>and</strong> right (O 0 2) of the mid-l<strong>in</strong>e<br />
(Hard<strong>in</strong>g et al., 1996). Electro-myographic activity (EMG)<br />
was recorded from two electrode pairs cover<strong>in</strong>g left <strong>and</strong><br />
right <strong>in</strong>dex ®nger extensor muscles. Electro-oculography<br />
(EOG) was recorded, <strong>in</strong> bipolar derivation, from electrodes<br />
placed at the outer canthi of both <strong>eye</strong>s. To control for <strong>eye</strong><br />
bl<strong>in</strong>ks <strong>and</strong> vertical <strong>eye</strong> <strong>movement</strong>s, an additional electrode<br />
was positioned above the nasion <strong>and</strong> referred to the electrode<br />
at the right outer canthus. EEG <strong>and</strong> EOG activity were<br />
ampli®ed with a b<strong>and</strong>-pass ®lter sett<strong>in</strong>g of 0.032±100 Hz;<br />
EMG was high-pass ®ltered at 5.2 Hz. Analog to digital<br />
conversion was performed at 256 Hz.<br />
2.4. Data analysis<br />
2.4.1. <strong>Motoric</strong> <strong>response</strong> latency<br />
For each subject, latencies of motor related activity<br />
follow<strong>in</strong>g Go stimuli <strong>and</strong> latencies of erroneously executed<br />
motor activity follow<strong>in</strong>g NoGo stimuli were determ<strong>in</strong>ed.<br />
<strong>Motoric</strong> <strong>response</strong> latency was de®ned as the time <strong>in</strong>terval<br />
between onset of the imperative change <strong>in</strong> stimulus colour<br />
<strong>and</strong> onset of motoric <strong>response</strong> activity. <strong>Motoric</strong> <strong>response</strong><br />
onset was determ<strong>in</strong>ed, off-l<strong>in</strong>e, by superimpos<strong>in</strong>g a vertical<br />
hairl<strong>in</strong>e cursor on the recorded EMG or EOG traces for<br />
®nger <strong>and</strong> <strong>eye</strong> <strong>movement</strong> conditions, respectively (Barrett<br />
et al., 1985). <strong>Motoric</strong> <strong>response</strong> latency was evaluated statistically<br />
by means of repeated measures analysis of variance<br />
(ANOVA). The analysis was performed with with<strong>in</strong>-subject<br />
variables Go/NoGo (Go trials vs. NoGo trials), Movement<br />
Modality (®nger extension vs. <strong>eye</strong> <strong>movement</strong>) <strong>and</strong> Movement<br />
Side (right vs. left ®nger extension, rightward vs. leftward<br />
<strong>eye</strong> <strong>movement</strong>).<br />
2.4.2. Event related potentials (ERPs)<br />
For each subject <strong>and</strong> <strong>movement</strong> condition, the stimulus,<br />
as well as <strong>response</strong> synchronized averaged ERP pro®les<br />
were constructed for Go <strong>and</strong> NoGo trials. Trials with artefacts<br />
<strong>in</strong> the ERPs, <strong>in</strong>clud<strong>in</strong>g <strong>eye</strong> <strong>movement</strong> artefacts, ampli-<br />
®er clipp<strong>in</strong>g <strong>and</strong> extensive EMG activity <strong>and</strong>/or electrophysiological<br />
drift, were rejected. Artefacts from required<br />
saccades <strong>in</strong> the <strong>eye</strong> <strong>movement</strong> conditions were corrected by<br />
means of a subtraction procedure described previously (Van<br />
`t Ent <strong>and</strong> Apkarian, 1998). Averages subtended from 3.25 s<br />
preced<strong>in</strong>g to 1 s follow<strong>in</strong>g stimulus completion or <strong>movement</strong><br />
onset. In the stimulus aligned epochs the ®rst 250 ms,