periodic - Lorentz JÄNTSCHI
periodic - Lorentz JÄNTSCHI
periodic - Lorentz JÄNTSCHI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
•<br />
•<br />
•<br />
•<br />
•<br />
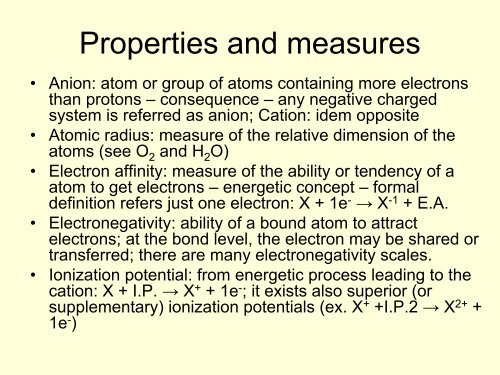
Properties and measures<br />
Anion: atom or group of atoms containing more electrons<br />
than protons – consequence – any negative charged<br />
system is referred as anion; Cation: idem opposite<br />
Atomic radius: measure of the relative dimension of the<br />
atoms (see O2 and H2O) Electron affinity: measure of the ability or tendency of a<br />
atom to get electrons – energetic concept – formal<br />
definition refers just one electron: X + 1e- → X-1 + E.A.<br />
Electronegativity: ability of a bound atom to attract<br />
electrons; at the bond level, the electron may be shared or<br />
transferred; there are many electronegativity scales.<br />
Ionization potential: from energetic process leading to the<br />
cation: X + I.P. → X + + 1e- ; it exists also superior (or<br />
supplementary) ionization potentials (ex. X + +I.P.2 → X2+ +<br />
1e- )