Autoignition Temperatures for Mixtures of Flammable Liquids with ...
Autoignition Temperatures for Mixtures of Flammable Liquids with ...
Autoignition Temperatures for Mixtures of Flammable Liquids with ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
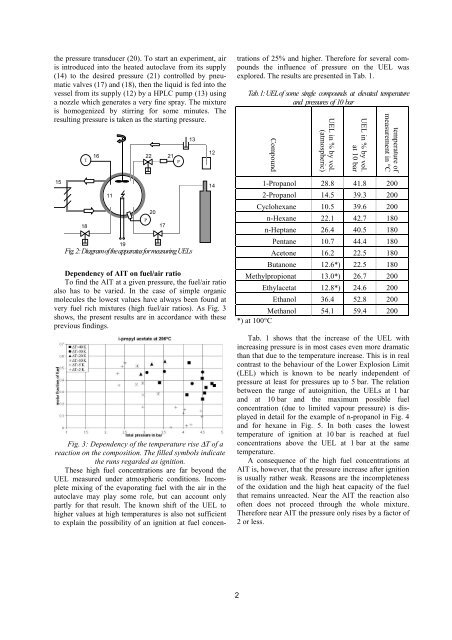
the pressure transducer (20). To start an experiment, air<br />
is introduced into the heated autoclave from its supply<br />
(14) to the desired pressure (21) controlled by pneumatic<br />
valves (17) and (18), then the liquid is fed into the<br />
vessel from its supply (12) by a HPLC pump (13) using<br />
a nozzle which generates a very fine spray. The mixture<br />
is homogenized by stirring <strong>for</strong> some minutes. The<br />
resulting pressure is taken as the starting pressure.<br />
15<br />
16<br />
22 21<br />
T P<br />
18<br />
11<br />
19<br />
Fig. 2: Diagram <strong>of</strong> the apparatus <strong>for</strong> measuring UELs<br />
Dependency <strong>of</strong> AIT on fuel/air ratio<br />
To find the AIT at a given pressure, the fuel/air ratio<br />
also has to be varied. In the case <strong>of</strong> simple organic<br />
molecules the lowest values have always been found at<br />
very fuel rich mixtures (high fuel/air ratios). As Fig. 3<br />
shows, the present results are in accordance <strong>with</strong> these<br />
previous findings.<br />
Fig. 3: Dependency <strong>of</strong> the temperature rise ΔT <strong>of</strong> a<br />
reaction on the composition. The filled symbols indicate<br />
the runs regarded as ignition.<br />
These high fuel concentrations are far beyond the<br />
UEL measured under atmospheric conditions. Incomplete<br />
mixing <strong>of</strong> the evaporating fuel <strong>with</strong> the air in the<br />
autoclave may play some role, but can account only<br />
partly <strong>for</strong> that result. The known shift <strong>of</strong> the UEL to<br />
higher values at high temperatures is also not sufficient<br />
to explain the possibility <strong>of</strong> an ignition at fuel concen-<br />
P<br />
20<br />
17<br />
13<br />
12<br />
14<br />
trations <strong>of</strong> 25% and higher. There<strong>for</strong>e <strong>for</strong> several compounds<br />
the influence <strong>of</strong> pressure on the UEL was<br />
explored. The results are presented in Tab. 1.<br />
2<br />
Tab. 1: UEL <strong>of</strong> some single compounds at elevated temperature<br />
and pressures <strong>of</strong> 10 bar<br />
Compound<br />
UEL in % by vol.<br />
(atmospheric)<br />
UEL in % by vol.<br />
at 10 bar<br />
temperature <strong>of</strong><br />
measurement in °C<br />
1-Propanol 28.8 41.8 200<br />
2-Propanol 14.5 39.3 200<br />
Cyclohexane 10.5 39.6 200<br />
n-Hexane 22.1 42.7 180<br />
n-Heptane 26.4 40.5 180<br />
Pentane 10.7 44.4 180<br />
Acetone 16.2 22.5 180<br />
Butanone 12.6*) 22.5 180<br />
Methylpropionat 13.0*) 26.7 200<br />
Ethylacetat 12.8*) 24.6 200<br />
Ethanol 36.4 52.8 200<br />
Methanol 54.1 59.4 200<br />
*) at 100°C<br />
Tab. 1 shows that the increase <strong>of</strong> the UEL <strong>with</strong><br />
increasing pressure is in most cases even more dramatic<br />
than that due to the temperature increase. This is in real<br />
contrast to the behaviour <strong>of</strong> the Lower Explosion Limit<br />
(LEL) which is known to be nearly independent <strong>of</strong><br />
pressure at least <strong>for</strong> pressures up to 5 bar. The relation<br />
between the range <strong>of</strong> autoignition, the UELs at 1 bar<br />
and at 10 bar and the maximum possible fuel<br />
concentration (due to limited vapour pressure) is displayed<br />
in detail <strong>for</strong> the example <strong>of</strong> n-propanol in Fig. 4<br />
and <strong>for</strong> hexane in Fig. 5. In both cases the lowest<br />
temperature <strong>of</strong> ignition at 10 bar is reached at fuel<br />
concentrations above the UEL at 1 bar at the same<br />
temperature.<br />
A consequence <strong>of</strong> the high fuel concentrations at<br />
AIT is, however, that the pressure increase after ignition<br />
is usually rather weak. Reasons are the incompleteness<br />
<strong>of</strong> the oxidation and the high heat capacity <strong>of</strong> the fuel<br />
that remains unreacted. Near the AIT the reaction also<br />
<strong>of</strong>ten does not proceed through the whole mixture.<br />
There<strong>for</strong>e near AIT the pressure only rises by a factor <strong>of</strong><br />
2 or less.