Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Plant</strong> <strong>cell</strong> <strong>wall</strong> (<strong>Lecture</strong> <strong>12</strong>)<br />
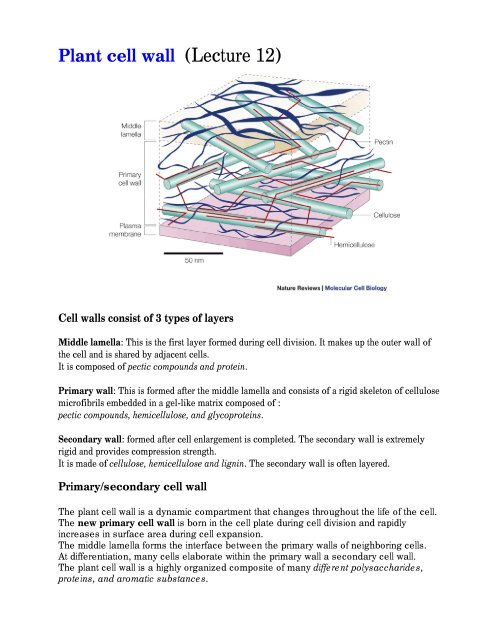
Cell <strong>wall</strong>s consist of 3 types of layers<br />
Middle lamella: This is the first layer formed during <strong>cell</strong> division. It makes up the outer <strong>wall</strong> of<br />
the <strong>cell</strong> and is shared by adjacent <strong>cell</strong>s.<br />
It is composed of pectic compounds and protein.<br />
Primary <strong>wall</strong>: This is formed after the middle lamella and consists of a rigid skeleton of <strong>cell</strong>ulose<br />
microfibrils embedded in a gel-like matrix composed of :<br />
pectic compounds, hemi<strong>cell</strong>ulose, and glycoproteins.<br />
Secondary <strong>wall</strong>: formed after <strong>cell</strong> enlargement is completed. The secondary <strong>wall</strong> is extremely<br />
rigid and provides compression strength.<br />
It is made of <strong>cell</strong>ulose, hemi<strong>cell</strong>ulose and lignin. The secondary <strong>wall</strong> is often layered.<br />
Primary/secondary <strong>cell</strong> <strong>wall</strong><br />
The plant <strong>cell</strong> <strong>wall</strong> is a dynamic compartment that changes throughout the life of the <strong>cell</strong>.<br />
The new primary <strong>cell</strong> <strong>wall</strong> is born in the <strong>cell</strong> plate during <strong>cell</strong> division and rapidly<br />
increases in surface area during <strong>cell</strong> expansion.<br />
The middle lamella forms the interface between the primary <strong>wall</strong>s of neighboring <strong>cell</strong>s.<br />
At differentiation, many <strong>cell</strong>s elaborate within the primary <strong>wall</strong> a secondary <strong>cell</strong> <strong>wall</strong>.<br />
The plant <strong>cell</strong> <strong>wall</strong> is a highly organized composite of many different polysaccharides,<br />
proteins, and aromatic substances.
Specialized functions of the <strong>cell</strong> <strong>wall</strong><br />
1.Structural:<br />
1.Fibers<br />
2.Cross-linked matrix<br />
2.Molecules affecting developmental pattern<br />
3.Molecules defining <strong>cell</strong>’s position within the plant.<br />
4.Molecules of <strong>cell</strong>-<strong>cell</strong> and <strong>cell</strong>-nucleus communication<br />
5.Defense against pathogens (impregnation with lignin)<br />
6.Recognition of symbiotic nitrogen-fixing bacteria<br />
7.Recognition of self<br />
Macromolecules of the <strong>cell</strong> <strong>wall</strong><br />
1.Cellulose – the most abundant plant polysacharide (15-30% of the dry mass of the <strong>cell</strong><br />
<strong>wall</strong>)<br />
Microfibrills – several dozen of (1à4) b-D-glucan chains<br />
Cellulose polymers associate through H-bonds.<br />
The H-bonding of many <strong>cell</strong>ulose molecules to each other results in the formation of micro fibers<br />
and the micro fibers can interact to form fibers.<br />
Certain <strong>cell</strong>s, like those in cotton ovules, can grow <strong>cell</strong>ulose fibers of enormous lengths.<br />
2. Callose<br />
Differ from <strong>cell</strong>ulose in consisting of (1à3) b-D-glucan chains that can form helical duplexes.<br />
Callose is made in a few <strong>cell</strong>s at specific stages of development (growing pollen tubes, <strong>cell</strong> plates<br />
of dividing <strong>cell</strong>s).
It is made in response to wounding or to penetration by invading fungal hyphae.<br />
3. Pectic acid<br />
- polymer of around 100 galacturonic acid molecules<br />
- very hydrophilic and soluble - become very hydrated<br />
- forms salts and salt bridges with Ca++ and Mg++ that are insoluble gels<br />
- major component of middle lamella but also found in primary <strong>wall</strong>s<br />
galacturonic acid Pectic acid with salt bridges<br />
4. Pectin<br />
- polymer of around 200 galacturonic acid molecules<br />
- many of the carboxyl groups are methylated (COOCH3)<br />
- less hydrated then pectic acid but soluble in hot water<br />
- another major component of middle lamella but also found in primary <strong>wall</strong>s<br />
Cross-linking glycans<br />
Make hydrogen-bond to <strong>cell</strong>ulose.<br />
•XyGs – xyloglucans<br />
•GAXs - glucuronoarabinoxylans<br />
Macromolecules of the <strong>cell</strong> <strong>wall</strong><br />
1.Structural proteins of the <strong>cell</strong> <strong>wall</strong><br />
i. Hydroxyproline-rich glycoproteins (HRGPs) Extensin
ii. Proline-rich glycoproteins (PRGPs)<br />
iii. Glycine-rich proteins (GRPs)<br />
iv. AGPs (mainly consist of carbohydrate)<br />
v. All mRNAs for <strong>cell</strong> <strong>wall</strong> proteins encode signal peptides that target the proteins to the<br />
secretory pathway.<br />
Like the <strong>cell</strong> <strong>wall</strong> carbohydrates, glycoproteins are hydrophilic and can form H-bonds and<br />
salt bridges with <strong>cell</strong> <strong>wall</strong> polysaccharides.<br />
In addition to hydroxyproline, <strong>cell</strong> <strong>wall</strong> proteins are often high in the amino acids proline<br />
and lysine. The NH3+ on lysine provides positive charges along the peptide backbone.<br />
The positive charges residues can associate with negatively charged groups on pectic<br />
acids, etc.<br />
In addition to electrostatic interactions, H-bonds also form between amino acid side<br />
chains and <strong>cell</strong> <strong>wall</strong> carbohydrates.<br />
Another type of structural <strong>cell</strong> <strong>wall</strong> protein, called extensin, can form covalent bonds with<br />
other extensin proteins through the amino acid tyrosine.<br />
In extensin, the tyrosines are evenly spaced and when they bond with tyrosine on another<br />
extensin molecule, they can wrap around other <strong>cell</strong> <strong>wall</strong> constituents "knitting" the <strong>wall</strong><br />
together.<br />
The amount of extensin changes with development. Cells that have thick, hard <strong>wall</strong>s are<br />
often rich in extensin (i.e., sclerids and fibers).<br />
The amount of extensin produced is dependent on mechanical wounding, infection and<br />
these responses are mediated by plant hormones.<br />
Cell <strong>wall</strong>s also contain functional proteins. Enzymatic activities in <strong>cell</strong> <strong>wall</strong>s include:<br />
•Oxidative enzymes - peroxidases•Hydrolytic enzymes - pectinases,<br />
<strong>cell</strong>ulases•"Expansins" - enzymes that catalyze <strong>cell</strong> <strong>wall</strong> "creep" activity<br />
General functions of <strong>cell</strong> <strong>wall</strong> enzymes include:<br />
protection against pathogens,<br />
<strong>cell</strong> expansion,<br />
<strong>cell</strong> <strong>wall</strong> maturation.<br />
Cell <strong>wall</strong> architecture<br />
The primary <strong>cell</strong> <strong>wall</strong> is made up of two, sometimes three, structurally independent but<br />
interacting networks.<br />
The framework of <strong>cell</strong>ulose and crosslinking glycans lies embedded in a second<br />
network of matrix pectic polysaccharides.
The third independent network consists of the structural proteins or phenylpropanoid<br />
network.<br />
Type I <strong>cell</strong> <strong>wall</strong>.<br />
The <strong>wall</strong>s of most dicots and the noncommelinoid monocots contain about equal<br />
amounts of XyGs and <strong>cell</strong>ulose<br />
Type II <strong>cell</strong> <strong>wall</strong>s<br />
Occurs in commelinoid monocots.<br />
Instead of XyG the principal polymers are GAXs<br />
•XyGs – xyloglucans<br />
•GAXs - glucuronoarabinoxylans<br />
Cell expansion involves extensive changes in the mass and composition of the <strong>cell</strong> <strong>wall</strong>.<br />
During elongation or expansion, existing <strong>cell</strong> <strong>wall</strong> architecture must change to incorporate<br />
new material, increasing the surface area of the <strong>cell</strong> and inducing water uptake by the<br />
protoplast.<br />
Wall loosening and continued deposition of new material - primary determinant of<br />
rates of <strong>cell</strong> expansion.<br />
The <strong>wall</strong>-localized event, called stress relaxation, serves as the fundamental difference<br />
between growing and nongrowing <strong>cell</strong>s.<br />
The yield threshold, defines the pressure potential that must be exceeded before<br />
expansion can occur.<br />
Postulates that auxin-dependent acidification of the <strong>cell</strong> <strong>wall</strong> promotes <strong>wall</strong> extensibility<br />
and <strong>cell</strong> growth.<br />
The acid-growth proposes that auxin activates a plasma-membrane proton pump, which<br />
acidifies the <strong>cell</strong> <strong>wall</strong>.<br />
The low pH activates apoplast-localized growth-specific hydrolases, which cleave the<br />
load-bearing bonds that tether <strong>cell</strong>ulose microfibrils to other polysaccharides.<br />
Cleavage of these bonds results in loosening of the <strong>cell</strong> <strong>wall</strong>, and the water potential<br />
difference causes uptake of water.<br />
Relaxation of the <strong>wall</strong> passively leads to an increase in <strong>cell</strong> size.<br />
Enzymes with <strong>wall</strong>-loosening acitivities<br />
Xyloglucan endotransglycosylase (XET) –<br />
transglycosylation of XyG in which one chain of XyG is cleaved and reattached to the<br />
nonreducing terminus of another XyG chain.<br />
Other proteins catalyze <strong>wall</strong> extension in vitro without any detectable hydrolytic or<br />
tansglycolytic events.<br />
Called expansins, these proteins probably catalyze breakage of hydrogen bonds<br />
between <strong>cell</strong>ulose and the load-bearing cross-linking glycans.<br />
Such an activity could disrupt the tethering of <strong>cell</strong>ulose.<br />
Expansins are the only proteins shown to produce <strong>wall</strong> expansion in vitro.<br />
They appear ubiquitous in growing tissues of all flowering plants, and they appear to<br />
break hydrogen bonds between <strong>cell</strong>ulose and cross-linking glycans.<br />
Cell <strong>wall</strong> loosening can occur by at least 3 mechanisms:
1) Wall acidification - H+ATPase in plasma membrane 'pumps" H+ from cytoplasm into<br />
<strong>cell</strong> <strong>wall</strong>.<br />
The pH of the <strong>wall</strong> drops and carboxylic acids become protonated and 'salt bridges"<br />
are broken.<br />
2) In addition, the enzyme "expansin" is activated and causes <strong>cell</strong>ulose micro fibers to slip<br />
(mechanism of expansin action is unknown).<br />
This results in <strong>cell</strong> <strong>wall</strong> "creep".<br />
3) Hydrolytic enzymes like <strong>cell</strong>ulase and pectinase, "degrade" <strong>cell</strong> <strong>wall</strong>s by breaking polymers<br />
into smaller subunits or by breaking crosslinks.<br />
Cell differentiation<br />
Within a single <strong>wall</strong> there are zones of different architectures – the middle lamella,<br />
plasmodesmata, thickenings, channels, pitfields, and the <strong>cell</strong> corners.<br />
The size of such microdomains in the <strong>wall</strong>, compared with the size of the polymers that<br />
must fit in these domains, implies that mechanisms must exist for packaging and<br />
positioning of large molecules.<br />
Changes of <strong>cell</strong> <strong>wall</strong> architecture are involved in fruit-ripening<br />
Most fruits in which the pericarp or endocarp softens during ripening develop thickened primary<br />
<strong>wall</strong>s (pectic substances)<br />
The texture of the ripe fruit pulp is governed by the extent of <strong>wall</strong> degradation and loss of <strong>cell</strong>-<strong>cell</strong><br />
adhesion.<br />
Pectin often constitute more than 50% of the fruit <strong>wall</strong>.<br />
The softening process in tomato parenhyma tissue is associated with loss of methyl esters of<br />
HGA (pectic polysaccharide homogalacturonan).<br />
The deesterified HGA backbone then is susceptible to the activity of Pgase<br />
(peptidoglutaminase).PGase solubilizes pectins from the <strong>cell</strong> <strong>wall</strong>, facilitating progressive<br />
hydrolysis.<br />
Pectin modification within the <strong>wall</strong> during ripening is a tightly regulated process.<br />
Using antisense inhibition of PGase, researchers were able almost completely preventing pectic<br />
depolymerization in transgenic fruit.<br />
However, little or no reduction in softening was achieved.<br />
For many <strong>cell</strong>s, the differentiation process is associated with formation of a secondary <strong>wall</strong>.<br />
When <strong>cell</strong>s stop growing, the <strong>wall</strong> is crosslinked into its ultimate shape. At that point, deposition of<br />
the secondary <strong>wall</strong> begins.<br />
Elaborate specialization of secondary <strong>wall</strong>s
Cotton fiber – 98% <strong>cell</strong>ulose at maturity.<br />
May contain additional non<strong>cell</strong>ulosic polysaccharides, proteins, and aromatic substances such as<br />
lignin.<br />
The <strong>wall</strong>s of many <strong>cell</strong>s function long after the <strong>cell</strong>s that produced them are dead.<br />
Secondary <strong>cell</strong> <strong>wall</strong>s of the cotyledon and the endosperm of the developing seeds contain little or<br />
no <strong>cell</strong>ulose.<br />
These secondary <strong>wall</strong>s serve two functions:<br />
•Provide a strong <strong>wall</strong> to protect the embryo or impose mechanical dormancy;<br />
•Contain specialized storage carbohydrates that are digested during germination and converted to<br />
sucrose for transport to the growing seedling.<br />
Approximately 1000 gene products involved in <strong>cell</strong> <strong>wall</strong> biosynthesis, assembly, and turnover are<br />
known.<br />
Arabidopsis mutants with altered carbohydrate components in the primary <strong>wall</strong> have been<br />
detected by gas chromatography of alditol acetates of their neutral sugars.<br />
More than three dozen mutants have been classified by mapping to 11 different loci.<br />
Mur1 has been identified as a GDP-mannose-4,6-dehydratase;<br />
Mur2 as a fucosyltransferase ;<br />
Mur4 – C-4 epimerase.<br />
Rsw1 - prymary <strong>wall</strong> <strong>cell</strong>ulose synthase.<br />
Cell <strong>wall</strong>s as food, feed, and fibers<br />
Cell <strong>wall</strong>s directly affect the raw material quality of human and animal food, textiles, wood, and<br />
paper and may play a role in human medicine.<br />
The food industry uses isolated arabinogalactan proteins (AGPs) and pectins as gums and gelling<br />
agents.<br />
Fungal and bacterial <strong>wall</strong> hydrolases are used to adjust food textures and states.<br />
The <strong>cell</strong> <strong>wall</strong>s of fruits and vegetables are now recognized as important dietary components and<br />
may protect against cancer of the colon, coronary heart disease, diabetes.<br />
b-Glucans are the causal agents in the ability of oat and barley brans to lower serum cholesterol<br />
and reduce the insulin demand of people with diabetes.<br />
Some pectins may have antitumor activities, possibly by stimulating immune system.<br />
Agricultural researches are investigating particular enzymes involved in <strong>cell</strong> <strong>wall</strong> metabolism in<br />
hopes of producing crops with desired characteristics by enhancing commercially valuable traits<br />
(fiber production in flax, cotton, ramie and sisal).<br />
The pulp and paper industry processes trees into <strong>cell</strong>ulose and livestock industry, which depends<br />
on the transformation of the <strong>cell</strong> <strong>wall</strong> into muscle tissue, are striving to reduce the lignin content in<br />
their respective sources of fiber and fodder.<br />
Reducing lignin content would reduce organochlorine wastes and cut costs tremendously for the<br />
paper industry, which currently uses chemical extractions to purify <strong>cell</strong>ulose from the wood.<br />
Lignin-carbohydrate interactions influence the digestibility of forage crops by animals.<br />
Hence, the mutants with altered lignin type may yield new forage crops that exhibit greater<br />
digestibility with remaining strengthening function of lignin to the water-conducting <strong>cell</strong>s of the<br />
plant.<br />
Unfortunately, the results of attempts to change only one parameter of <strong>wall</strong> metabolism are rarely<br />
those predicted, because of the complexity of the <strong>wall</strong> and the ability of plant <strong>cell</strong>s to adapt to<br />
change.
Summary<br />
1.The primary <strong>wall</strong> of the <strong>cell</strong> is extensible but constrains the final size and shape of every <strong>cell</strong><br />
2.In some <strong>cell</strong>s, secondary <strong>wall</strong>s are deposited on the inner surface of the primary <strong>wall</strong> after<br />
growth has stopped.<br />
3.Cell <strong>wall</strong> becomes specialized for the function of the approximately 40 <strong>cell</strong> types that plants<br />
comprise.<br />
4.The <strong>cell</strong>ulose microfibrills form the scaffold of all <strong>cell</strong> <strong>wall</strong>s and are tethered together by<br />
crosslinking glycans; this framework is embedded in a gel of pectic substances.<br />
5. There are two types of primary <strong>cell</strong> <strong>wall</strong>s:<br />
•Type I – xyloglucan-<strong>cell</strong>ulose networks embedded in pectin-rich matrix that is further cross-linked<br />
with a network of structural proteins.<br />
•Type II – have glycoronoarabinoxylan – <strong>cell</strong>ulose networks in a relatively pectin-poor matrix that<br />
is crosslinked by ferulate esters and aromatic substances.<br />
6.Cellulose microfibrills are synthesized at the surface of the plasma membrane at terminal<br />
complexes called particle rosettes. All non<strong>cell</strong>ulosic crosslinking glycans and pectic substances<br />
are made at the Golgi apparatus and secreted.<br />
7.All <strong>cell</strong> <strong>wall</strong> sugars are synthesized de novo from interconversion of nucleotide sugars<br />
8.Cell enlargement depends on the activities of endoglycosidase, endotransglycosylase, or<br />
expansin, or some combination of these; <strong>cell</strong> shape is largely governed by the pattern of <strong>cell</strong>ulose<br />
deposition.<br />
9. Termination of <strong>cell</strong> growth is accompanied by crosslinking reactions involving proteins and<br />
aromatic substances.<br />
10. Transgenic plants with altered <strong>cell</strong> <strong>wall</strong> structures will become an important factor in crop and<br />
biomass improvement.<br />
Part of the material was modified from<br />
•http://www.landfood.unimelb.edu.au/research/Biotech/research.html<br />
•http://www.agrevo.com/is_agrevo/biotech/ps/ps_sl_sl1.htm<br />
•http://www.regional.org.au/au/gcirc/4/354.htm