Chem1000A Spring 2007 Practice Assignment 6 - Answers
Chem1000A Spring 2007 Practice Assignment 6 - Answers
Chem1000A Spring 2007 Practice Assignment 6 - Answers
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
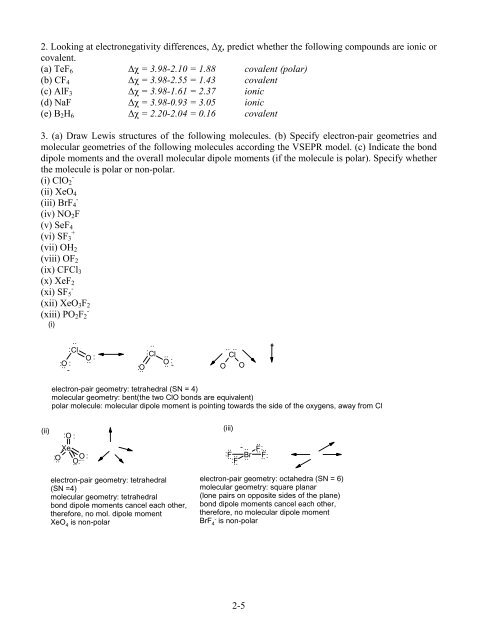
2. Looking at electronegativity differences, ∆χ, predict whether the following compounds are ionic or<br />
covalent.<br />
(a) TeF6 ∆χ = 3.98-2.10 = 1.88 covalent (polar)<br />
(b) CF4 ∆χ = 3.98-2.55 = 1.43 covalent<br />
(c) AlF3 ∆χ = 3.98-1.61 = 2.37 ionic<br />
(d) NaF ∆χ = 3.98-0.93 = 3.05 ionic<br />
(e) B2H6 ∆χ = 2.20-2.04 = 0.16 covalent<br />
3. (a) Draw Lewis structures of the following molecules. (b) Specify electron-pair geometries and<br />
molecular geometries of the following molecules according the VSEPR model. (c) Indicate the bond<br />
dipole moments and the overall molecular dipole moments (if the molecule is polar). Specify whether<br />
the molecule is polar or non-polar.<br />
(i) ClO2 -<br />
(ii) XeO4<br />
(iii) BrF4 -<br />
(iv) NO2F<br />
(v) SeF4<br />
(vi) SF3 +<br />
(vii) OH2<br />
(viii) OF2<br />
(ix) CFCl3<br />
(x) XeF2<br />
(xi) SF5 -<br />
(xii) XeO3F2<br />
(xiii) PO2F2 -<br />
(ii)<br />
(i)<br />
..<br />
: Cl<br />
: O..<br />
:<br />
-<br />
: O :<br />
Xe<br />
O O<br />
O..<br />
.. : :<br />
.. :<br />
O..<br />
:<br />
..<br />
..<br />
.. +<br />
: Cl ..<br />
Cl<br />
O :<br />
: O<br />
.. -<br />
O O<br />
..<br />
electron-pair geometry: tetrahedral (SN = 4)<br />
molecular geometry: bent(the two ClO bonds are equivalent)<br />
polar molecule: molecular dipole moment is pointing towards the side of the oxygens, away from Cl<br />
electron-pair geometry: tetrahedral<br />
(SN =4)<br />
molecular geometry: tetrahedral<br />
bond dipole moments cancel each other,<br />
therefore, no mol. dipole moment<br />
XeO 4 is non-polar<br />
(iii)<br />
..<br />
.. - .. F..<br />
: ..<br />
: F..<br />
.. Br .. F<br />
F<br />
.. :<br />
: ..<br />
electron-pair geometry: octahedra (SN = 6)<br />
molecular geometry: square planar<br />
(lone pairs on opposite sides of the plane)<br />
bond dipole moments cancel each other,<br />
therefore, no molecular dipole moment<br />
BrF 4 - is non-polar<br />
2-5