Physiological and molecular determinants of embryo implantation
Physiological and molecular determinants of embryo implantation
Physiological and molecular determinants of embryo implantation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
14 S. Zhang et al. / Molecular Aspects <strong>of</strong> Medicine xxx (2013) xxx–xxx<br />
carbohydrates, including L-selectin, are dispensable for <strong>implantation</strong> in mice, although they are expressed in the uterine epithelial<br />
cells (Domino et al., 2001).<br />
E-cadherin, a Ca 2+ -dependent transmembrane adhesion molecule, mediates intercellular adhesion <strong>and</strong> dynamic changes<br />
to the cytoskeleton through its interaction with cytoplasmic catenins (Goodrich <strong>and</strong> Strutt, 2011). E-cadherin is a critical factor<br />
for blastocyst formation, since <strong>embryo</strong>s lacking the E-cadherin gene fail to establish adhesion junctions in the trophectoderm<br />
<strong>and</strong> die in the peri<strong>implantation</strong> period (De Vries et al., 2004; Larue et al., 1994). On the maternal side, E-cadherin is<br />
highly expressed in the luminal epithelium prior to <strong>implantation</strong>, but is transiently downregulated before blastocyst invasion<br />
into the stroma, suggesting that remodeling the adhesion junctions between epithelial cells is a critical event during<br />
<strong>embryo</strong> <strong>implantation</strong> (Paria et al., 1999c; Thie et al., 1996, 1995, 1998). There is evidence that loosening <strong>of</strong> cell–cell junctions<br />
in the mouse uterine epithelium through downregulation <strong>of</strong> E-cadherin is a prerequisite for blastocyst attachment (Li et al.,<br />
2002; Thie et al., 1996). Indeed, E-cadherin is persistently expressed in the luminal epithelium <strong>of</strong> uterine-specific Msx1/Msx2<br />
ablated mice which show <strong>implantation</strong> failure (Daikoku et al., 2011; Nallasamy et al., 2012). Similarly, a recent observation<br />
shows that the expression <strong>of</strong> E-cadherin is remarkably downregulated in endometrial epithelial cells <strong>of</strong> the mid-secretory<br />
endometrium in women with endometriosis (Matsuzaki et al., 2010). Uterine-specific deletion <strong>of</strong> E-cadherin results in female<br />
infertility due to defective <strong>implantation</strong> <strong>and</strong> decidualization. The deficient mice lose adhesion junctions <strong>and</strong> tight junctions<br />
in the uterine epithelium, creating a disorganized cellular structure that is incapable <strong>of</strong> supporting <strong>embryo</strong> attachment<br />
<strong>and</strong> invasion (Reardon et al., 2012). Collectively, these findings indicate that E-cadherin plays critical roles during <strong>embryo</strong>nic<br />
<strong>and</strong> uterine preparation for <strong>implantation</strong>.<br />
5.3. The epithelial–stromal interaction<br />
Uterine tissue consists <strong>of</strong> three major layers: an outer muscle layer, the inner luminal epithelium <strong>and</strong> the stromal bed in<br />
between (Wang <strong>and</strong> Dey, 2006). Synchronization <strong>of</strong> estrogen <strong>and</strong> progesterone directs the uterus into the receptive state,<br />
accompanied by morphological <strong>and</strong> functional changes in the epithelium <strong>and</strong> the stroma. Increasing attention has been paid<br />
to underst<strong>and</strong> how these two hormones execute their differential effects on the two major endometrial cell types, <strong>and</strong> the<br />
<strong>molecular</strong> basis <strong>of</strong> stromal–epithelial interactions essential for uterine receptivity (Cooke et al., 1986; Cunha et al., 2004;<br />
Kurita et al., 2000b). The synergistic <strong>and</strong> antagonistic interactions <strong>of</strong> ovarian progesterone <strong>and</strong> estrogen on uterine cell proliferation<br />
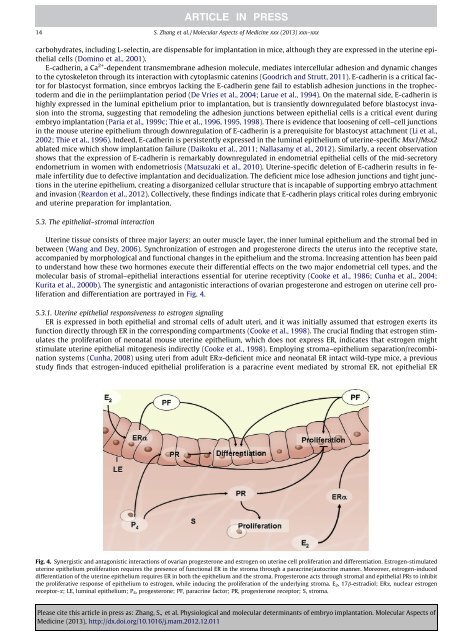
<strong>and</strong> differentiation are portrayed in Fig. 4.<br />
5.3.1. Uterine epithelial responsiveness to estrogen signaling<br />
ER is expressed in both epithelial <strong>and</strong> stromal cells <strong>of</strong> adult uteri, <strong>and</strong> it was initially assumed that estrogen exerts its<br />
function directly through ER in the corresponding compartments (Cooke et al., 1998). The crucial finding that estrogen stimulates<br />
the proliferation <strong>of</strong> neonatal mouse uterine epithelium, which does not express ER, indicates that estrogen might<br />
stimulate uterine epithelial mitogenesis indirectly (Cooke et al., 1998). Employing stroma–epithelium separation/recombination<br />
systems (Cunha, 2008) using uteri from adult ERa-deficient mice <strong>and</strong> neonatal ER intact wild-type mice, a previous<br />
study finds that estrogen-induced epithelial proliferation is a paracrine event mediated by stromal ER, not epithelial ER<br />
Fig. 4. Synergistic <strong>and</strong> antagonistic interactions <strong>of</strong> ovarian progesterone <strong>and</strong> estrogen on uterine cell proliferation <strong>and</strong> differentiation. Estrogen-stimulated<br />
uterine epithelium proliferation requires the presence <strong>of</strong> functional ER in the stroma through a paracrine/autocrine manner. Moreover, estrogen-induced<br />
differentiation <strong>of</strong> the uterine epithelium requires ER in both the epithelium <strong>and</strong> the stroma. Progesterone acts through stromal <strong>and</strong> epithelial PRs to inhibit<br />
the proliferative response <strong>of</strong> epithelium to estrogen, while inducing the proliferation <strong>of</strong> the underlying stroma. E 2,17b-estradiol; ERa, nuclear estrogen<br />
receptor-a; LE, luminal epithelium; P 4, progesterone; PF, paracrine factor; PR, progesterone receptor; S, stroma.<br />
Please cite this article in press as: Zhang, S., et al. <strong>Physiological</strong> <strong>and</strong> <strong>molecular</strong> <strong>determinants</strong> <strong>of</strong> <strong>embryo</strong> <strong>implantation</strong>. Molecular Aspects <strong>of</strong><br />
Medicine (2013), http://dx.doi.org/10.1016/j.mam.2012.12.011