Physics 9 Fall 2011 Homework 4 - Solutions Friday September 16 ...
Physics 9 Fall 2011 Homework 4 - Solutions Friday September 16 ...
Physics 9 Fall 2011 Homework 4 - Solutions Friday September 16 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Physics</strong> 9 <strong>Fall</strong> <strong>2011</strong><br />
<strong>Homework</strong> 4 - <strong>Solutions</strong><br />
<strong>Friday</strong> <strong>September</strong> <strong>16</strong>, <strong>2011</strong><br />
Make sure your name is on your homework, and please box your final answer. Because<br />
we will be giving partial credit, be sure to attempt all the problems, even if you don’t finish<br />
them. The homework is due at the beginning of class on <strong>Friday</strong>, <strong>September</strong> 23rd. Because<br />
the solutions will be posted immediately after class, no late homeworks can be accepted! You<br />
are welcome to ask questions during the discussion session or during office hours.<br />
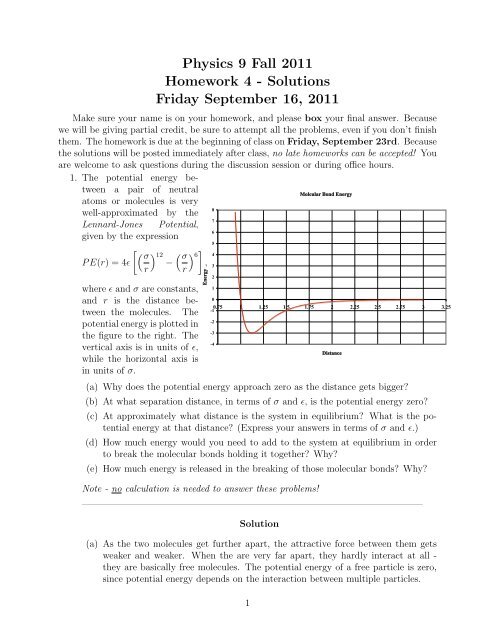
1. The potential energy between<br />
a pair of neutral<br />
atoms or molecules is very<br />
Molcular Bond Energy<br />
well-approximated by the<br />
8<br />
Lennard-Jones Potential,<br />
7<br />
given by the expression<br />
6<br />
5<br />
σ 12 <br />
σ<br />
<br />
6 4<br />
P E(r) = 4ɛ − , 3<br />
r r<br />
where ɛ and σ are constants,<br />
and r is the distance between<br />
the molecules. The<br />
potential energy is plotted in<br />
the figure to the right. The<br />
vertical axis is in units of ɛ,<br />
while the horizontal axis is<br />
in units of σ.<br />
Energy<br />
2<br />
1<br />
0<br />
0.75 1 1.25 1.5 1.75 2 2.25 2.5 2.75 3 3.25<br />
-1<br />
-2<br />
-3<br />
-4<br />
Distance<br />
(a) Why does the potential energy approach zero as the distance gets bigger?<br />
(b) At what separation distance, in terms of σ and ɛ, is the potential energy zero?<br />
(c) At approximately what distance is the system in equilibrium? What is the potential<br />
energy at that distance? (Express your answers in terms of σ and ɛ.)<br />
(d) How much energy would you need to add to the system at equilibrium in order<br />
to break the molecular bonds holding it together? Why?<br />
(e) How much energy is released in the breaking of those molecular bonds? Why?<br />
Note - no calculation is needed to answer these problems!<br />
————————————————————————————————————<br />
Solution<br />
(a) As the two molecules get further apart, the attractive force between them gets<br />
weaker and weaker. When the are very far apart, they hardly interact at all -<br />
they are basically free molecules. The potential energy of a free particle is zero,<br />
since potential energy depends on the interaction between multiple particles.<br />
1
(b) We can just read the value off from the graph. We see that the potential energy<br />
crosses the x axis when x = 1, which means that r = σ. We can see this from the<br />
equation, too: setting r = σ gives P E(σ) = 0.<br />
(c) The system is in equilibrium when the net force on it is zero. Since the force is<br />
the slope of the potential energy graph, this happens when the slope is zero. The<br />
potential energy graph has zero slope when it’s at it’s minimum point. Checking<br />
the graph, we see that this happens right around x ≈ 1.15, or r ≈ 1.15σ. We could<br />
check the exact answer by finding d<br />
dr (P E(r)) = 0, which gives r = 21/6 σ ≈ 1.12σ,<br />
and so we were close on our guess. The energy at this distance can just be read<br />
off the graph, giving y = −3, or P E = −3ɛ.<br />
(d) In order to break the molecular bonds apart, we’d need to raise the energy to<br />
zero. At equilibrium the energy is P E = −3ɛ, and so we’d need to add +3ɛ units<br />
of energy.<br />
(e) There is no energy released in breaking these molecular bonds - we had to add<br />
the energy to break these bonds. Energy is never released in the breaking of<br />
bonds! One can obtain energy by breaking a less stable bond, then forming a<br />
more stable bond. The more stable bond has a more negative potential energy<br />
(a deeper potential “well”). The difference in energy between the initial and final<br />
states is released to the environment. This is where the energy comes from in the<br />
ATP reactions, and not by releasing energy from the breaking of bonds!<br />
2
2. In a potassium chloride molecule, the distance between the potassium ion (K + ) and<br />
the chloride ion (Cl − ) is 2.80 × 10 −10 m.<br />
(a) Calculate the energy (in Joules and eV) required to separate the two ions to an<br />
infinite distance apart (i.e., to break the bonds holding the molecule together).<br />
(Model the two ions as two point particles initially at rest.)<br />
(b) If twice the energy determined in part (a) is actually supplied, what is the total<br />
amount of kinetic energy that the two ions have when they were an infinite<br />
distance apart?<br />
————————————————————————————————————<br />
Solution<br />
(a) The potential energy of the molecule is just the electrostatic energy,<br />
P E = − 1<br />
4πɛ0<br />
qQ<br />
r ,<br />
where q and Q are the two charges, and r is their separation distance. The net<br />
charge on each ion is ±e, the electron charge. Plugging in the numbers gives<br />
P E = − 1<br />
4πɛ0<br />
qQ<br />
r = −9 × 109 × (1.602 × 10−19 ) 2<br />
2.80 × 10−10 = −8.25 × 10 −19 Joules.<br />
One Joule is 1.602 × 10 −19 eV, so the potential energy in electron volts is P E =<br />
−5.15 eV. This potential energy is the energy released when the two ions bonded,<br />
and to break these bonds we would need to add the exact same amount of energy.<br />
Thus, the energy required to break the bonds and separate the two ions to an<br />
infinite distance is 5.15 electron volts.<br />
(b) The minimum energy needed to break the molecule is 5.15 eV, which just separates<br />
the ions with no final kinetic energy. So, any additional energy put into breaking<br />
the bonds would go into the kinetic energy of the two ions. Thus, if we put in<br />
an extra 5.15 eV of energy, then this will be the total kinetic energy of the ions<br />
when they are infinitely separated.<br />
3
3. Calculate the potential inside and outside a sphere of radius R and charge Q, in which<br />
the the charge is distributed uniformly throughout the sphere. (Hint: recall the electric<br />
fields inside and outside a uniformly charged sphere from Gauss’s law, and don’t forget<br />
that the potentials must be continuous at the surface of the sphere, where r = R.)<br />
————————————————————————————————————<br />
Solution<br />
The electric field outside a uniformly charged sphere is just the ordinary Coulomb law,<br />
Eout = 1<br />
4πɛ0<br />
Q<br />
,<br />
r2 while inside, as we have seen from Gauss’s law, the field is<br />
Ein = 1<br />
4πɛ0<br />
Q<br />
r.<br />
R3 Now, the potential outside the sphere is simply the electrostatic potential of a point<br />
charge,<br />
Vout = 1 Q<br />
4πɛ0 r .<br />
The potential inside the sphere is a bit harder. We can find it by integrating the<br />
electric field, looking for the total work done in bringing in a unit charge from infinity<br />
and placing it at some position inside the sphere. Thus,<br />
r<br />
Vin(r) = −<br />
∞<br />
R r<br />
E · ds = − Eout · ds −<br />
∞<br />
R<br />
Ein · ds,<br />
where we have split up the integral because the electric field is different in the two<br />
regions. So, plugging in the fields and integrating over radial distance, r, gives<br />
Vin(r) = − Q<br />
4πɛ0<br />
R<br />
∞<br />
dr Q<br />
−<br />
r2 4πɛ0R3 r<br />
r dr =<br />
R<br />
1 Q 1<br />
−<br />
4πɛ0 R 4πɛ0<br />
Q<br />
2R3 2 2<br />
r − R .<br />
Simplifying and combining the terms gives our final result for the potential,<br />
Vin(r) = Q<br />
8πɛ0R<br />
Note that Vin(R) = Vout(R), as we require.<br />
4<br />
<br />
3 − r2<br />
R 2<br />
<br />
.
4. A solid sphere of radius R has a uniform charge density ρ and total charge Q. Derive an<br />
expression for its total electric potential energy in terms of R and Q. (Hint: Imagine<br />
that the sphere is constructed by adding successive layers of concentric shells of charge<br />
dq = (4πr 2 dr) ρ and use dU = V dq.)<br />
————————————————————————————————————<br />
Solution<br />
Suppose that the sphere starts out as a ball of radius r and charge q. Then, the voltage<br />
of the ball is<br />
V (r) = 1 q<br />
4πɛ0 r .<br />
The work required to bring in a tiny charge dq and place it on the surface of the ball<br />
is dU = V dq, or<br />
dU = 1 qdq<br />
4πɛ0 r .<br />
Now, we can’t directly integrate this to get the total potential energy because the<br />
radius doesn’t stay fixed - it builds up to a final radius of R. So, we need to express<br />
the charge in terms of the radius. We know that q = 4π<br />
3 r3 ρ, such that dq = 4πr 2 drρ,<br />
where the charge density is constant. Thus,<br />
dU = 1<br />
4πɛ0<br />
(4π) 2 ρ 2<br />
3<br />
r 4 dr.<br />
Now we can integrate this result, building the radius up from zero to R,<br />
U = 1 (4π)<br />
4πɛ0<br />
2 ρ2 R<br />
r<br />
3 0<br />
4 dr = 1<br />
4πɛ0<br />
(4π) 2 ρ 2 R 5<br />
We can express this result in terms of the total charge Q recalling that ρ = Q/Vol =<br />
3Q<br />
4πR 3 , and so we finally find<br />
U = 1 (4π)<br />
4πɛ0<br />
2 ρ2R5 15<br />
5<br />
= 1<br />
4πɛ0<br />
9Q 2 R 5<br />
15R<br />
15<br />
3 Q<br />
= 6 5<br />
2<br />
4πɛ0R .<br />
.
5. When an uncharged conducting sphere of radius a is placed at the origin of an xyz<br />
coordinate system that lies in an initially uniform electric field E = E0 ˆ k, the resulting<br />
electrostatic potential is V (x, y, z) = V0 for points inside the sphere, and<br />
V (x, y, z) = V0 − E0z +<br />
E0a 3 z<br />
(x 2 + y 2 + z 2 ) 3/2<br />
for points outside the sphere, where V0 is the (constant) electrostatic potential on the<br />
conductor. Use this equation to determine the x, y, and z components of the resulting<br />
electric field. What is the full electric field in vector notation?<br />
————————————————————————————————————<br />
Solution<br />
The electric field may be found from the potential by taking the gradient,<br />
such that the components are Ex = − ∂V<br />
need to take the partial derivatives.<br />
E = −∇V,<br />
So, the x and y components are straightforward,<br />
Ex = − ∂V<br />
∂x = −E0a 3 z ∂<br />
<br />
1<br />
∂x<br />
and<br />
Ey = − ∂V<br />
∂y = −E0a 3 z ∂<br />
<br />
∂y<br />
∂x , Ey = − ∂V<br />
∂y , and Ez = − ∂V<br />
∂z<br />
(x 2 + y 2 + z 2 ) 3/2<br />
1<br />
(x 2 + y 2 + z 2 ) 3/2<br />
The z component is more work, but not too much more.<br />
Ez = − ∂V<br />
<br />
∂<br />
= −<br />
∂z ∂z<br />
V0 − E0z +<br />
E0a3 <br />
z<br />
= E0−<br />
(x 2 + y 2 + z 2 ) 3/2<br />
<br />
<br />
=<br />
=<br />
3E0a 3 zx<br />
(x2 + y2 + z2 , 5/2<br />
)<br />
3E0a 3 zy<br />
(x2 + y2 + z2 . 5/2<br />
)<br />
E0a 3<br />
(x 2 + y 2 + z 2 )<br />
. Thus, we just<br />
3/2 +<br />
3E0a3z2 (x2 + y2 + z2 . 5/2<br />
)<br />
This is all of the components, and we can write the full electric field in component<br />
form as<br />
E = −∇V = − ∂V ∂V<br />
î −<br />
∂x ∂y ˆj − ∂V<br />
∂z ˆ k,<br />
such that<br />
E =<br />
3E0a 3 zx<br />
(x 2 + y 2 + z 2 )<br />
5/2 î+<br />
3E0a 3 zy<br />
(x2 + y2 + z2 ˆj+ 5/2<br />
)<br />
6<br />
<br />
E0 −<br />
E0a 3<br />
(x2 + y2 + z2 + 3/2<br />
)<br />
3E0a 3 z 2<br />
(x 2 + y 2 + z 2 ) 5/2<br />
<br />
ˆk.