Paper-and-Glue Unit Cell Models W
Paper-and-Glue Unit Cell Models W
Paper-and-Glue Unit Cell Models W
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chemistry for Everyone<br />
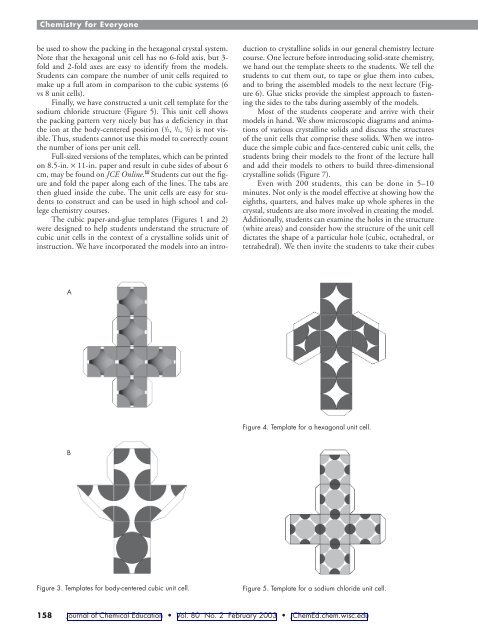
be used to show the packing in the hexagonal crystal system.<br />
Note that the hexagonal unit cell has no 6-fold axis, but 3fold<br />
<strong>and</strong> 2-fold axes are easy to identify from the models.<br />
Students can compare the number of unit cells required to<br />
make up a full atom in comparison to the cubic systems (6<br />
vs 8 unit cells).<br />
Finally, we have constructed a unit cell template for the<br />
sodium chloride structure (Figure 5). This unit cell shows<br />
the packing pattern very nicely but has a deficiency in that<br />
the ion at the body-centered position ( 1 /2, 1 /2, 1 /2) is not visible.<br />
Thus, students cannot use this model to correctly count<br />
the number of ions per unit cell.<br />
Full-sized versions of the templates, which can be printed<br />
on 8.5-in. × 11-in. paper <strong>and</strong> result in cube sides of about 6<br />
cm, may be found on JCE Online. W Students cut out the figure<br />
<strong>and</strong> fold the paper along each of the lines. The tabs are<br />
then glued inside the cube. The unit cells are easy for students<br />
to construct <strong>and</strong> can be used in high school <strong>and</strong> college<br />
chemistry courses.<br />
The cubic paper-<strong>and</strong>-glue templates (Figures 1 <strong>and</strong> 2)<br />
were designed to help students underst<strong>and</strong> the structure of<br />
cubic unit cells in the context of a crystalline solids unit of<br />
instruction. We have incorporated the models into an intro-<br />
A<br />
B<br />
Figure 3. Templates for body-centered cubic unit cell.<br />
duction to crystalline solids in our general chemistry lecture<br />
course. One lecture before introducing solid-state chemistry,<br />
we h<strong>and</strong> out the template sheets to the students. We tell the<br />
students to cut them out, to tape or glue them into cubes,<br />
<strong>and</strong> to bring the assembled models to the next lecture (Figure<br />
6). <strong>Glue</strong> sticks provide the simplest approach to fastening<br />
the sides to the tabs during assembly of the models.<br />
Most of the students cooperate <strong>and</strong> arrive with their<br />
models in h<strong>and</strong>. We show microscopic diagrams <strong>and</strong> animations<br />
of various crystalline solids <strong>and</strong> discuss the structures<br />
of the unit cells that comprise these solids. When we introduce<br />
the simple cubic <strong>and</strong> face-centered cubic unit cells, the<br />
students bring their models to the front of the lecture hall<br />
<strong>and</strong> add their models to others to build three-dimensional<br />
crystalline solids (Figure 7).<br />
Even with 200 students, this can be done in 5–10<br />
minutes. Not only is the model effective at showing how the<br />
eighths, quarters, <strong>and</strong> halves make up whole spheres in the<br />
crystal, students are also more involved in creating the model.<br />
Additionally, students can examine the holes in the structure<br />
(white areas) <strong>and</strong> consider how the structure of the unit cell<br />
dictates the shape of a particular hole (cubic, octahedral, or<br />
tetrahedral). We then invite the students to take their cubes<br />
Figure 4. Template for a hexagonal unit cell.<br />
Figure 5. Template for a sodium chloride unit cell.<br />
158 Journal of Chemical Education • Vol. 80 No. 2 February 2003 • JChemEd.chem.wisc.edu