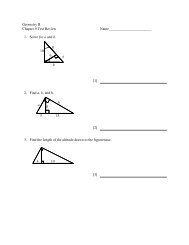
Ch. 20 Chemistry Exam Review
Ch. 20 Chemistry Exam Review
Ch. 20 Chemistry Exam Review
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Ch</strong>. <strong>20</strong> <strong>Ch</strong>emistry <strong>Exam</strong> <strong>Review</strong><br />
1. When zinc oxide becomes zinc, what type of reaction occurs?<br />
2. Define oxidation.<br />
3. In the reaction of lithium with oxygen, which atom is reduced?<br />
4. In the reaction of calcium with bromine, which atom is the reducing agent?<br />
5. What is the oxidizing agent in the following reaction?<br />
2Na + 2H O → 2NaOH + H<br />
6. What is the reducing agent in the following reaction?<br />
CH + 2O CO + H O<br />
7. What is oxidized and what is reduced in the following equation?<br />
S + Cl SCl<br />
8. Why is oxygen reduced in the reaction of hydrogen with oxygen to make water?<br />
9. The oxidation number of sodium in sodium chloride is ____.<br />
10. The oxidation number of nitrogen in nitrogen gas is ____.<br />
11. What is the oxidation number for each atom in NH4Cl?<br />
12. What is the oxidation number of sulfur in each of the following?<br />
a. SO b. Na SO c. SO d. S O<br />
13. What is the oxidation number of nitrogen in each of the following?<br />
a. NO b. N O c. NH Cl d. Ca(NO )<br />
14. Identify the atom that decreases in oxidation number in the following redox reaction.<br />
2MnO + 2K CO + O 2KMnO + 2CO<br />
15. In the following unbalanced reaction, which atom is oxidized?<br />
H O + Cl + SO HCl + H SO
16. In the following unbalanced reaction, which atom is reduced?<br />
HNO + HBr → NO + Br + H O<br />
17. Which element increases its oxidation number in the following reaction?<br />
BiCl + Na SO → 2NaCl + BiSO<br />
18. Which types of reactions are not redox reactions?<br />
19. Balance the following half-reaction.<br />
MnO → Mn<br />
<strong>20</strong>. What is the oxidation half-reaction for the following unbalanced redox equation?<br />
Cr O + NH → Cr O + N<br />
21. What is the reduction half-reaction for the following unbalanced redox equation?<br />
Cr O + Fe → Cr + Fe<br />
22. What is shown by a half-reaction?<br />
23. How can you identify if a half-reaction represents oxidation or reduction?<br />
24. When the half-reactions Br + 2e → 2Br and Na → Na + e are combined, what will the balanced redox<br />
equation be?<br />
25. Which type of reaction does Pb → Pb represent?