Cervical Cancer Screening and Human Papillomavirus (HPV ... - KCE
Cervical Cancer Screening and Human Papillomavirus (HPV ... - KCE
Cervical Cancer Screening and Human Papillomavirus (HPV ... - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
14 <strong>Cervical</strong> cancer screening <strong>and</strong> <strong>HPV</strong> <strong>KCE</strong> reports vol.38<br />
Both study design have significant limitations. With split-sample studies, it is difficult to<br />
ensure that the two cytology specimens are comparable <strong>and</strong> this design would seem to<br />
lead to bias against LBC since only the material remnant on the sampling device after<br />
preparation of a conventional smear can be used for LBC. It is possible that some<br />
diagnostic elements included in the CP-split sample are not available anymore for the<br />
LBC. In the two-cohort design it has been argued that the historical controls introduce<br />
other biases as the comparability of the populations being compared <strong>and</strong> expectation<br />
bias.<br />
The other major limitations found in most of the studies evaluating LBC are the lack of<br />
comparison of test performance with a gold st<strong>and</strong>ard ( blinded colposcopy/biopsy) <strong>and</strong><br />
study population of women followed-up for a previous abnormal test result rather than<br />
women undergoing routine screening. Large, r<strong>and</strong>omized controlled clinical trials need<br />
to be conducted. One large r<strong>and</strong>omized trial is currently ongoing in The Netherl<strong>and</strong>s<br />
but the results are not yet available.<br />
2.4.2 Performance<br />
2.4.2.1 Test positivity rate of cytological abnormalities<br />
Hutchinson, 1991<br />
Hutchinson, 1992<br />
Awen, 1994<br />
Wilbur, 1994<br />
Aponte-C, 1995<br />
Bur, 1995<br />
Laverty, 1995<br />
Sheets, 1995<br />
Ferenczy, 1996<br />
McGoogan, 1996<br />
Tezuka, 1996<br />
Wilbur, 1996<br />
Lee, 1997<br />
Roberts, 1997<br />
Corkill, 1998<br />
Boman, 1999<br />
Hutchinson, 1999<br />
Shield, 1999<br />
Wang, 1999<br />
Monsonego, 2001<br />
Park, 2001<br />
Armstrong, 2002<br />
Biscotti, 2002<br />
Grace, 2002<br />
Luthra, 2002<br />
Ring, 2002<br />
Coste, 2003<br />
Farnsworth, 2003<br />
Harkness, 2003<br />
Malle, 2003<br />
Masumoto, 2003<br />
Confortini, 2004<br />
Combined<br />
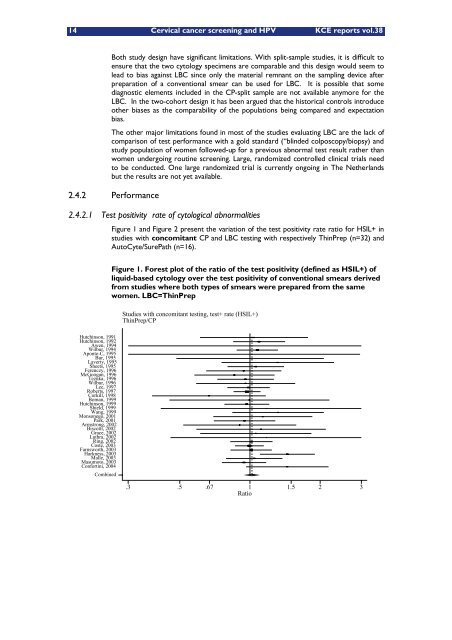
Figure 1 <strong>and</strong> Figure 2 present the variation of the test positivity rate ratio for HSIL+ in<br />
studies with concomitant CP <strong>and</strong> LBC testing with respectively ThinPrep (n=32) <strong>and</strong><br />
AutoCyte/SurePath (n=16).<br />
Figure 1. Forest plot of the ratio of the test positivity (defined as HSIL+) of<br />
liquid-based cytology over the test positivity of conventional smears derived<br />
from studies where both types of smears were prepared from the same<br />
women. LBC=ThinPrep<br />
Studies with concomitant testing, test+ rate (HSIL+)<br />
ThinPrep/CP<br />
.3 .5 .67 1 1.5 2 3<br />
Ratio