The reference price system and socioeconomic differences in ... - KCE
The reference price system and socioeconomic differences in ... - KCE
The reference price system and socioeconomic differences in ... - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
20 Reference Price System <strong>KCE</strong> reports 126<br />
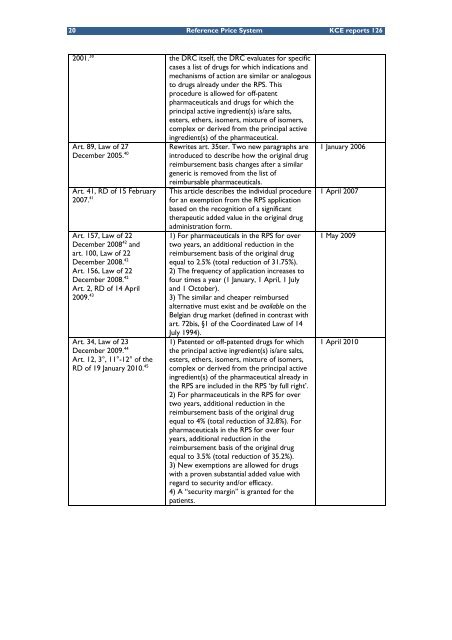
2001. 38 the DRC itself, the DRC evaluates for specific<br />
cases a list of drugs for which <strong>in</strong>dications <strong>and</strong><br />
mechanisms of action are similar or analogous<br />
to drugs already under the RPS. This<br />
procedure is allowed for off-patent<br />
pharmaceuticals <strong>and</strong> drugs for which the<br />
pr<strong>in</strong>cipal active <strong>in</strong>gredient(s) is/are salts,<br />
esters, ethers, isomers, mixture of isomers,<br />
complex or derived from the pr<strong>in</strong>cipal active<br />
Art. 89, Law of 27<br />
December 2005. 40<br />
Art. 41, RD of 15 February<br />
2007. 41<br />
Art. 157, Law of 22<br />
December 2008 42 <strong>and</strong><br />
art. 100, Law of 22<br />
December 2008. 42<br />
Art. 156, Law of 22<br />
December 2008. 42<br />
Art. 2, RD of 14 April<br />
2009. 43<br />
Art. 34, Law of 23<br />
December 2009. 44<br />
Art. 12, 3°, 11°-12° of the<br />
RD of 19 January 2010. 45<br />
<strong>in</strong>gredient(s) of the pharmaceutical.<br />
Rewrites art. 35ter. Two new paragraphs are<br />
<strong>in</strong>troduced to describe how the orig<strong>in</strong>al drug<br />
reimbursement basis changes after a similar<br />
generic is removed from the list of<br />
reimbursable pharmaceuticals.<br />
This article describes the <strong>in</strong>dividual procedure<br />
for an exemption from the RPS application<br />
based on the recognition of a significant<br />
therapeutic added value <strong>in</strong> the orig<strong>in</strong>al drug<br />
adm<strong>in</strong>istration form.<br />
1) For pharmaceuticals <strong>in</strong> the RPS for over<br />
two years, an additional reduction <strong>in</strong> the<br />
reimbursement basis of the orig<strong>in</strong>al drug<br />
equal to 2.5% (total reduction of 31.75%).<br />
2) <strong>The</strong> frequency of application <strong>in</strong>creases to<br />
four times a year (1 January, 1 April, 1 July<br />
<strong>and</strong> 1 October).<br />
3) <strong>The</strong> similar <strong>and</strong> cheaper reimbursed<br />
alternative must exist <strong>and</strong> be available on the<br />
Belgian drug market (def<strong>in</strong>ed <strong>in</strong> contrast with<br />
art. 72bis, §1 of the Coord<strong>in</strong>ated Law of 14<br />
July 1994).<br />
1) Patented or off-patented drugs for which<br />
the pr<strong>in</strong>cipal active <strong>in</strong>gredient(s) is/are salts,<br />
esters, ethers, isomers, mixture of isomers,<br />
complex or derived from the pr<strong>in</strong>cipal active<br />
<strong>in</strong>gredient(s) of the pharmaceutical already <strong>in</strong><br />
the RPS are <strong>in</strong>cluded <strong>in</strong> the RPS ‘by full right’.<br />
2) For pharmaceuticals <strong>in</strong> the RPS for over<br />
two years, additional reduction <strong>in</strong> the<br />
reimbursement basis of the orig<strong>in</strong>al drug<br />
equal to 4% (total reduction of 32.8%). For<br />
pharmaceuticals <strong>in</strong> the RPS for over four<br />
years, additional reduction <strong>in</strong> the<br />
reimbursement basis of the orig<strong>in</strong>al drug<br />
equal to 3.5% (total reduction of 35.2%).<br />
3) New exemptions are allowed for drugs<br />
with a proven substantial added value with<br />
regard to security <strong>and</strong>/or efficacy.<br />
4) A “security marg<strong>in</strong>” is granted for the<br />
patients.<br />
1 January 2006<br />
1 April 2007<br />
1 May 2009<br />
1 April 2010