MODELING CHAR OXIDATION AS A FUNCTION OF PRESSURE ...
MODELING CHAR OXIDATION AS A FUNCTION OF PRESSURE ...
MODELING CHAR OXIDATION AS A FUNCTION OF PRESSURE ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
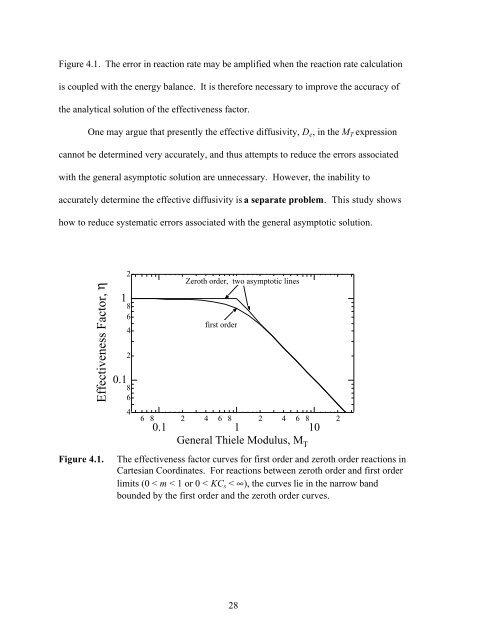
Figure 4.1. The error in reaction rate may be amplified when the reaction rate calculation<br />
is coupled with the energy balance. It is therefore necessary to improve the accuracy of<br />
the analytical solution of the effectiveness factor.<br />
One may argue that presently the effective diffusivity, D e, in the M T expression<br />
cannot be determined very accurately, and thus attempts to reduce the errors associated<br />
with the general asymptotic solution are unnecessary. However, the inability to<br />
accurately determine the effective diffusivity is a separate problem. This study shows<br />
how to reduce systematic errors associated with the general asymptotic solution.<br />
Effectiveness Factor, η<br />
2<br />
1<br />
8<br />
6<br />
4<br />
2<br />
0.1<br />
8<br />
6<br />
4<br />
6 8<br />
0.1<br />
Zeroth order, two asymptotic lines<br />
first order<br />
2 4 6 8<br />
1<br />
General Thiele Modulus, MT 28<br />
2 4 6 8<br />
10<br />
Figure 4.1. The effectiveness factor curves for first order and zeroth order reactions in<br />
Cartesian Coordinates. For reactions between zeroth order and first order<br />
limits (0 < m < 1 or 0 < KC s < ∞), the curves lie in the narrow band<br />
bounded by the first order and the zeroth order curves.<br />
2