A Transferable Force Field To Predict Phase Equilibria and Surface ...
A Transferable Force Field To Predict Phase Equilibria and Surface ...
A Transferable Force Field To Predict Phase Equilibria and Surface ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
The Journal of Physical Chemistry B ARTICLE<br />
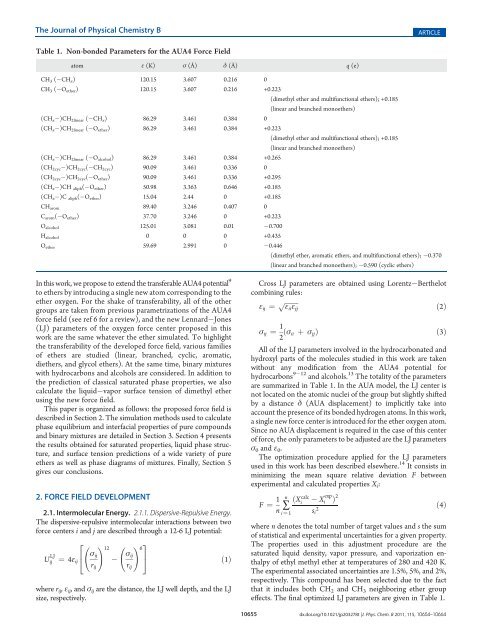
Table 1. Non-bonded Parameters for the AUA4 <strong>Force</strong> <strong>Field</strong><br />
In this work, we propose to extend the transferable AUA4 potential 9<br />
to ethers by introducing a single new atom corresponding to the<br />
ether oxygen. For the shake of transferability, all of the other<br />
groups are taken from previous parametrizations of the AUA4<br />
force field (see ref 6 for a review), <strong>and</strong> the new Lennard Jones<br />
(LJ) parameters of the oxygen force center proposed in this<br />
work are the same whatever the ether simulated. <strong>To</strong> highlight<br />
the transferability of the developed force field, various families<br />
of ethers are studied (linear, branched, cyclic, aromatic,<br />
diethers, <strong>and</strong> glycol ethers). At the same time, binary mixtures<br />
with hydrocarbons <strong>and</strong> alcohols are considered. In addition to<br />
the prediction of classical saturated phase properties, we also<br />
calculate the liquid vapor surface tension of dimethyl ether<br />
using the new force field.<br />
This paper is organized as follows: the proposed force field is<br />
described in Section 2. The simulation methods used to calculate<br />
phase equilibrium <strong>and</strong> interfacial properties of pure compounds<br />
<strong>and</strong> binary mixtures are detailed in Section 3. Section 4 presents<br />
the results obtained for saturated properties, liquid phase structure,<br />
<strong>and</strong> surface tension predictions of a wide variety of pure<br />
ethers as well as phase diagrams of mixtures. Finally, Section 5<br />
gives our conclusions.<br />
2. FORCE FIELD DEVELOPMENT<br />
2.1. Intermolecular Energy. 2.1.1. Dispersive-Repulsive Energy.<br />
The dispersive-repulsive intermolecular interactions between two<br />
force centers i <strong>and</strong> j are described through a 12-6 LJ potential:<br />
U LJ<br />
ij ¼ 4εij<br />
atom ε (K) σ (Å) δ (Å) q (e)<br />
CH3 ( CHx) 120.15 3.607 0.216 0<br />
CH3 ( Oether) 120.15 3.607 0.216 +0.223<br />
(dimethyl ether <strong>and</strong> multifunctional ethers); +0.185<br />
(linear <strong>and</strong> branched monoethers)<br />
(CHx )CH2linear ( CHx) 86.29 3.461 0.384 0<br />
(CHx )CH2linear ( Oether) 86.29 3.461 0.384 +0.223<br />
(dimethyl ether <strong>and</strong> multifunctional ethers); +0.185<br />
(linear <strong>and</strong> branched monoethers)<br />
(CH x )CH 2linear ( O alcohol) 86.29 3.461 0.384 +0.265<br />
(CH2cyc )CH2cyc( CH2cyc) 90.09 3.461 0.336 0<br />
(CH2cyc )CH2cyc( Oether) 90.09 3.461 0.336 +0.295<br />
(CHx )CH aliph( Oether) 50.98 3.363 0.646 +0.185<br />
(CHx )C aliph( Oether) 15.04 2.44 0 +0.185<br />
CHarom 89.40 3.246 0.407 0<br />
Carom( Oether) 37.70 3.246 0 +0.223<br />
Oalcohol 125.01 3.081 0.01 0.700<br />
Halcohol 0 0 0 +0.435<br />
Oether 59.69 2.991 0 0.446<br />
(dimethyl ether, aromatic ethers, <strong>and</strong> multifunctional ethers); 0.370<br />
(linear <strong>and</strong> branched monoethers); 0.590 (cyclic ethers)<br />
2<br />
4<br />
σij<br />
rij<br />
! 12<br />
σij<br />
rij<br />
! 6<br />
3<br />
5 ð1Þ<br />
where rij, εij,<strong>and</strong>σij are the distance, the LJ well depth, <strong>and</strong> the LJ<br />
size, respectively.<br />
Cross LJ parameters are obtained using Lorentz<br />
combining rules:<br />
Berthelot<br />
εij ¼ ffiffiffiffiffiffiffiffi p<br />
εiiεjj<br />
ð2Þ<br />
σij ¼ 1<br />
2 ðσii þ σjjÞ ð3Þ<br />
All of the LJ parameters involved in the hydrocarbonated <strong>and</strong><br />
hydroxyl parts of the molecules studied in this work are taken<br />
without any modification from the AUA4 potential for<br />
hydrocarbons 9 12 <strong>and</strong> alcohols. 13 The totality of the parameters<br />
are summarized in Table 1. In the AUA model, the LJ center is<br />
not located on the atomic nuclei of the group but slightly shifted<br />
by a distance δ (AUA displacement) to implicitly take into<br />
account the presence of its bonded hydrogen atoms. In this work,<br />
a single new force center is introduced for the ether oxygen atom.<br />
Since no AUA displacement is required in the case of this center<br />
of force, the only parameters to be adjusted are the LJ parameters<br />
σ0 <strong>and</strong> ε0.<br />
The optimization procedure applied for the LJ parameters<br />
used in this work has been described elsewhere. 14 It consists in<br />
minimizing the mean square relative deviation F between<br />
experimental <strong>and</strong> calculated properties Xi:<br />
F ¼ 1<br />
n ∑n<br />
ðX<br />
i ¼ 1<br />
calc<br />
i<br />
X exp<br />
i Þ 2<br />
si 2<br />
10655 dx.doi.org/10.1021/jp203278t |J. Phys. Chem. B 2011, 115, 10654–10664<br />
ð4Þ<br />
where n denotes the total number of target values <strong>and</strong> s the sum<br />
of statistical <strong>and</strong> experimental uncertainties for a given property.<br />
The properties used in this adjustment procedure are the<br />
saturated liquid density, vapor pressure, <strong>and</strong> vaporization enthalpy<br />
of ethyl methyl ether at temperatures of 280 <strong>and</strong> 420 K.<br />
The experimental associated uncertainties are 1.5%, 5%, <strong>and</strong> 2%,<br />
respectively. This compound has been selected due to the fact<br />
that it includes both CH2 <strong>and</strong> CH3 neighboring ether group<br />
effects. The final optimized LJ parameters are given in Table 1.