A novel 8.7 kDa protease inhibitor from chan seeds (Hyptis ... - UAM
A novel 8.7 kDa protease inhibitor from chan seeds (Hyptis ... - UAM
A novel 8.7 kDa protease inhibitor from chan seeds (Hyptis ... - UAM
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
84 C. Aguirre et al. / Comparative Biochemistry and Physiology Part B 138 (2004) 81–89<br />
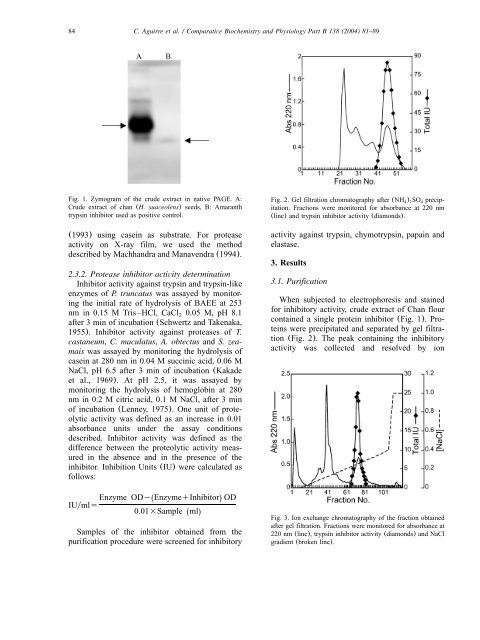
Fig. 1. Zymogram of the crude extract in native PAGE. A:<br />
Crude extract of <strong>chan</strong> (H. suaveolens) <strong>seeds</strong>, B: Amaranth<br />
trypsin <strong>inhibitor</strong> used as positive control.<br />
(1993) using casein as substrate. For <strong>protease</strong><br />
activity on X-ray film, we used the method<br />
described by Machhandra and Manavendra (1994).<br />
2.3.2. Protease <strong>inhibitor</strong> activity determination<br />
Inhibitor activity against trypsin and trypsin-like<br />
enzymes of P. truncatus was assayed by monitoring<br />
the initial rate of hydrolysis of BAEE at 253<br />
nm in 0.15 M Tris–HCl, CaCl2<br />
0.05 M, pH 8.1<br />
after 3 min of incubation (Schwertz and Takenaka,<br />
1955). Inhibitor activity against <strong>protease</strong>s of T.<br />
castaneum, C. maculatus, A. obtectus and S. zeamais<br />
was assayed by monitoring the hydrolysis of<br />
casein at 280 nm in 0.04 M succinic acid, 0.06 M<br />
NaCl, pH 6.5 after 3 min of incubation (Kakade<br />
et al., 1969). At pH 2.5, it was assayed by<br />
monitoring the hydrolysis of hemoglobin at 280<br />
nm in 0.2 M citric acid, 0.1 M NaCl, after 3 min<br />
of incubation (Lenney, 1975). One unit of proteolytic<br />
activity was defined as an increase in 0.01<br />
absorbance units under the assay conditions<br />
described. Inhibitor activity was defined as the<br />
difference between the proteolytic activity measured<br />
in the absence and in the presence of the<br />
<strong>inhibitor</strong>. Inhibition Units (IU) were calculated as<br />
follows:<br />
IUymls<br />
Enzyme ODyŽ EnzymeqInhibitor.<br />
OD<br />
0.01=Sample<br />
Ž ml.<br />
Samples of the <strong>inhibitor</strong> obtained <strong>from</strong> the<br />
purification procedure were screened for <strong>inhibitor</strong>y<br />
Fig. 2. Gel filtration chromatography after (NH ) SO precip-<br />
4 2 4<br />
itation. Fractions were monitored for absorbance at 220 nm<br />
(line) and trypsin <strong>inhibitor</strong> activity (diamonds).<br />
activity against trypsin, chymotrypsin, papain and<br />
elastase.<br />
3. Results<br />
3.1. Purification<br />
When subjected to electrophoresis and stained<br />
for <strong>inhibitor</strong>y activity, crude extract of Chan flour<br />
contained a single protein <strong>inhibitor</strong> (Fig. 1). Proteins<br />
were precipitated and separated by gel filtration<br />
(Fig. 2). The peak containing the <strong>inhibitor</strong>y<br />
activity was collected and resolved by ion<br />
Fig. 3. Ion ex<strong>chan</strong>ge chromatography of the fraction obtained<br />
after gel filtration. Fractions were monitored for absorbance at<br />
220 nm (line), trypsin <strong>inhibitor</strong> activity (diamonds) and NaCl<br />
gradient (broken line).