Extending the Van Laar Model to ... - Bentham Science
Extending the Van Laar Model to ... - Bentham Science
Extending the Van Laar Model to ... - Bentham Science
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
136 The Open Thermodynamics Journal, 2010, Volume 4 Ding-Yu Peng<br />
21<br />
35<br />
19<br />
30<br />
P/kPa<br />
17<br />
15<br />
P/kPa<br />
25<br />
13<br />
20<br />
11<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
15<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
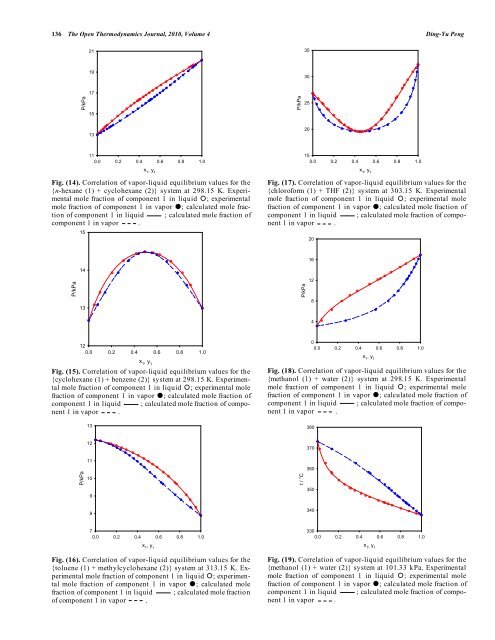
Fig. (14). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{n-hexane (1) + cyclohexane (2)} system at 298.15 K. Experimental<br />
mole fraction of component 1 in liquid ; experimental<br />
mole fraction of component 1 in vapor ; calculated mole fraction<br />
of component 1 in liquid ; calculated mole fraction of<br />
component 1 in vapor .<br />
15<br />
Fig. (17). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{chloroform (1) + THF (2)} system at 303.15 K. Experimental<br />
mole fraction of component 1 in liquid ; experimental mole<br />
fraction of component 1 in vapor ; calculated mole fraction of<br />
component 1 in liquid ; calculated mole fraction of component<br />
1 in vapor .<br />
20<br />
16<br />
14<br />
P/kPa<br />
13<br />
P/kPa<br />
12<br />
8<br />
4<br />
12<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
Fig. (15). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{cyclohexane (1) + benzene (2)} system at 298.15 K. Experimental<br />
mole fraction of component 1 in liquid ; experimental mole<br />
fraction of component 1 in vapor ; calculated mole fraction of<br />
component 1 in liquid ; calculated mole fraction of component<br />
1 in vapor .<br />
13<br />
0<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
Fig. (18). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{methanol (1) + water (2)} system at 298.15 K. Experimental<br />
mole fraction of component 1 in liquid ; experimental mole<br />
fraction of component 1 in vapor ; calculated mole fraction of<br />
component 1 in liquid ; calculated mole fraction of component<br />
1 in vapor .<br />
380<br />
12<br />
370<br />
P/kPa<br />
11<br />
10<br />
9<br />
t / ο C<br />
360<br />
350<br />
8<br />
340<br />
7<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
Fig. (16). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{<strong>to</strong>luene (1) + methylcyclohexane (2)} system at 313.15 K. Experimental<br />
mole fraction of component 1 in liquid ; experimental<br />
mole fraction of component 1 in vapor ; calculated mole<br />
fraction of component 1 in liquid ; calculated mole fraction<br />
of component 1 in vapor .<br />
330<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x 1<br />
, y 1<br />
Fig. (19). Correlation of vapor-liquid equilibrium values for <strong>the</strong><br />
{methanol (1) + water (2)} system at 101.33 kPa. Experimental<br />
mole fraction of component 1 in liquid ; experimental mole<br />
fraction of component 1 in vapor ; calculated mole fraction of<br />
component 1 in liquid ; calculated mole fraction of component<br />
1 in vapor .