p18ctqouf5gk614re1k6k1lc31s0l4.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
154 Rates of Chemical Weathering<br />
36<br />
32<br />
28 UNTREATED<br />
:2<br />
-<br />
'0 24<br />
U/<br />
0 20<br />
bI 16<br />
0<br />
E 12<br />
::!.<br />
Z 8<br />
0<br />
~<br />
en<br />
4<br />
00 20 40 60 80<br />
Time (days)<br />
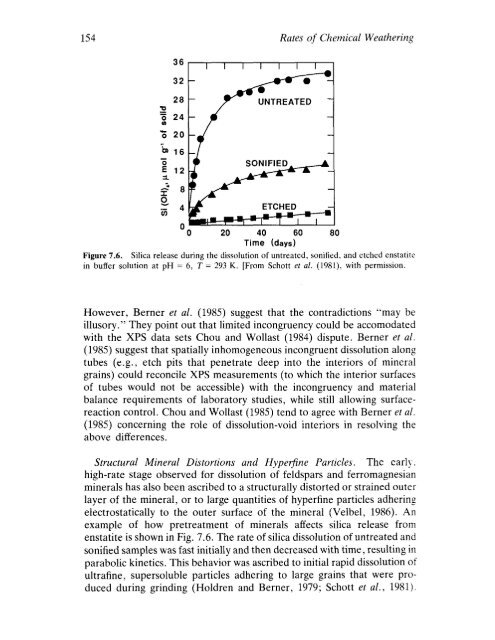
Figure 7.6. Silica release during the dissolution of untreated, sonified, and etched enstatite<br />
in buffer solution at pH = 6, T = 293 K. [From Schott et al. (1981), with permission.<br />
However, Berner et al. (1985) suggest that the contradictions "may be<br />
illusory." They point out that limited incongruency could be accomodated<br />
with the XPS data sets Chou and Wollast (1984) dispute. Berner et al.<br />
(1985) suggest that spatially inhomogeneous incongruent dissolution along<br />
tubes (e.g., etch pits that penetrate deep into the interiors of mineral<br />
grains) could reconcile XPS measurements (to which the interior surfaces<br />
of tubes would not be accessible) with the incongruency and material<br />
balance requirements of laboratory studies, while still allowing surfacereaction<br />
control. Chou and Wollast (1985) tend to agree with Berner et al.<br />
(1985) concerning the role of dissolution-void interiors in resolving the<br />
above differences.<br />
Structural Mineral Distortions and Hyperfine Particles. The early.<br />
high-rate stage observed for dissolution of feldspars and ferromagnesian<br />
minerals has also been ascribed to a structurally distorted or strained outer<br />
layer of the mineral, or to large quantities of hyperfine particles adhering<br />
electrostatically to the outer surface of the mineral (Velbel, 1986). An<br />
example of how pretreatment of minerals affects silica release from<br />
enstatite is shown in Fig. 7.6. The rate of silica dissolution of untreated and<br />
sonified samples was fast initially and then decreased with time, resulting in<br />
parabolic kinetics. This behavior was ascribed to initial rapid dissolution of<br />
uitrafine, supersoluble particles adhering to large grains that were produced<br />
during grinding (Holdren and Berner, 1979; Schott et al., 1981).