p18ctqouf5gk614re1k6k1lc31s0l4.pdf
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Oxidation Rates of Cations by Mn( Ill/IV) Oxides 171<br />
~<br />
20.0<br />
0 275K<br />
0<br />
E<br />
::t<br />
15.0<br />
[(5 -Mn02]o = 250 mg 1- 1<br />
"'C<br />
CII<br />
E ....<br />
[Cr{III)]o = 24.0 Jlmol I - 1<br />
0<br />
u. 10.0<br />
pH = 5.5<br />
~<br />
><br />
~<br />
....<br />
u 5.0<br />
10 20 30 40<br />
Time (min)<br />
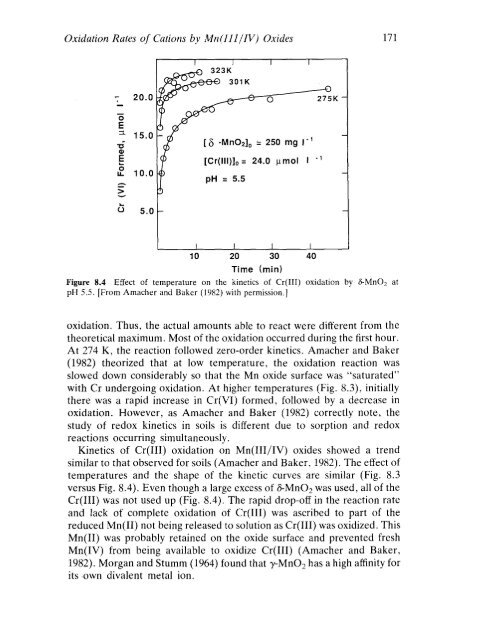
Figure 8.4 Effect of temperature on the kinetics of Cr(III) oxidation by 8-Mn02 at<br />
pH 5.5. [From Amacher and Baker (1982) with permission.]<br />
oxidation. Thus, the actual amounts able to react were different from the<br />
theoretical maximum. Most of the oxidation occurred during the first hour.<br />
At 274 K, the reaction followed zero-order kinetics. Amacher and Baker<br />
(1982) theorized that at low temperature, the oxidation reaction was<br />
slowed down considerably so that the Mn oxide surface was "saturated"<br />
with Cr undergoing oxidation. At higher temperatures (Fig. 8.3), initially<br />
there was a rapid increase in Cr(VI) formed, followed by a decrease in<br />
oxidation. However, as Amacher and Baker (1982) correctly note, the<br />
study of redox kinetics in soils is different due to sorption and redox<br />
reactions occurring simultaneously.<br />
Kinetics of Cr(IlI) oxidation on Mn(III/IV) oxides showed a trend<br />
similar to that observed for soils (Amacher and Baker, 1982). The effect of<br />
temperatures and the shape of the kinetic curves are similar (Fig. 8.3<br />
versus Fig. 8.4). Even though a large excess of 8-Mn02 was used, all of the<br />
Cr(IlI) was not used up (Fig. 8.4). The rapid drop-off in the reaction rate<br />
and lack of complete oxidation of Cr(IlI) was ascribed to part of the<br />
reduced Mn(lI) not being released to solution as Cr(IlI) was oxidized. This<br />
Mn(lI) was probably retained on the oxide surface and prevented fresh<br />
Mn(IV) from being available to oxidize Cr(IlI) (Amacher and Baker,<br />
1982). Morgan and Stumm (1964) found that y-Mn02 has a high affinity for<br />
its ·own divalent metal ion.