Alkohole - Chempage.de
Alkohole - Chempage.de
Alkohole - Chempage.de
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
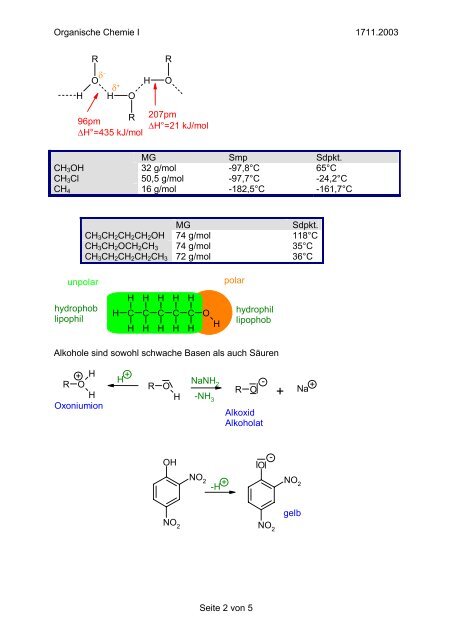
Organische Chemie I 1711.2003<br />
R<br />
O<br />
H H O<br />
H<br />
R<br />
O<br />
δ − δ + 207pm<br />
96pm<br />
R<br />
∆H°=435 kJ/mol<br />
∆H°=21 kJ/mol<br />
MG Smp Sdpkt.<br />
CH 3 OH 32 g/mol -97,8°C 65°C<br />
CH 3 Cl 50,5 g/mol -97,7°C -24,2°C<br />
CH 4 16 g/mol -182,5°C -161,7°C<br />
MG<br />
Sdpkt.<br />
CH 3 CH 2 CH 2 CH 2 OH 74 g/mol 118°C<br />
CH 3 CH 2 OCH 2 CH 3 74 g/mol 35°C<br />
CH 3 CH 2 CH 2 CH 2 CH 3 72 g/mol 36°C<br />
unpolar<br />
polar<br />
hydrophob<br />
lipophil<br />
H<br />
H H H H H<br />
C C C C C O<br />
H H H H H<br />
H<br />
hydrophil<br />
lipophob<br />
<strong>Alkohole</strong> sind sowohl schwache Basen als auch Säuren<br />
+ H +<br />
H<br />
R O<br />
H<br />
Oxoniumion<br />
R<br />
O<br />
H<br />
NaNH 2<br />
-NH 3<br />
R<br />
O<br />
-<br />
Alkoxid<br />
Alkoholat<br />
+<br />
Na<br />
+<br />
OH<br />
O -<br />
NO 2<br />
NO<br />
-H + 2<br />
gelb<br />
NO 2 NO 2<br />
Seite 2 von 5