Lecture PowerPoints Chapter 13 Physics: Principles with ...
Lecture PowerPoints Chapter 13 Physics: Principles with ...
Lecture PowerPoints Chapter 13 Physics: Principles with ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
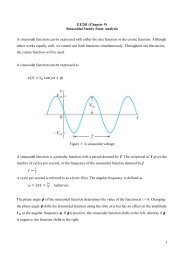
<strong>13</strong>-9 Ideal Gas Law in Terms of Molecules:<br />
Avogadro’s Number<br />
Since the gas constant is universal, the<br />
number of molecules in one mole is the same<br />
for all gases. That number is called Avogadro’s<br />
number:<br />
The number of molecules in a gas is the<br />
number of moles times Avogadro’s number: