Endorsed scope - Queensland Health
Endorsed scope - Queensland Health
Endorsed scope - Queensland Health
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
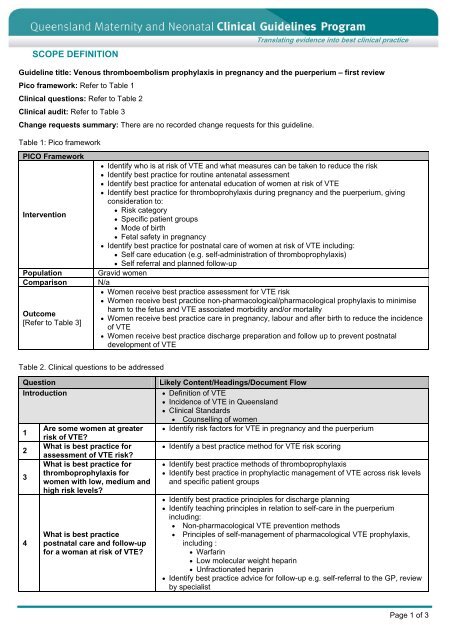
SCOPE DEFINITION<br />
Translating evidence into best clinical practice<br />
Guideline title: Venous thromboembolism prophylaxis in pregnancy and the puerperium – first review<br />
Pico framework: Refer to Table 1<br />
Clinical questions: Refer to Table 2<br />
Clinical audit: Refer to Table 3<br />
Change requests summary: There are no recorded change requests for this guideline.<br />
Table 1: Pico framework<br />
PICO Framework<br />
Identify who is at risk of VTE and what measures can be taken to reduce the risk<br />
Identify best practice for routine antenatal assessment<br />
Identify best practice for antenatal education of women at risk of VTE<br />
Identify best practice for thromboprohylaxis during pregnancy and the puerperium, giving<br />
consideration to:<br />
Risk category<br />
Intervention<br />
Specific patient groups<br />
Mode of birth<br />
Fetal safety in pregnancy<br />
Identify best practice for postnatal care of women at risk of VTE including:<br />
Self care education (e.g. self-administration of thromboprophylaxis)<br />
Self referral and planned follow-up<br />
Population Gravid women<br />
Comparison N/a<br />
Women receive best practice assessment for VTE risk<br />
Women receive best practice non-pharmacological/pharmacological prophylaxis to minimise<br />
harm to the fetus and VTE associated morbidity and/or mortality<br />
Outcome<br />
Women receive best practice care in pregnancy, labour and after birth to reduce the incidence<br />
[Refer to Table 3]<br />
of VTE<br />
Women receive best practice discharge preparation and follow up to prevent postnatal<br />
development of VTE<br />
Table 2. Clinical questions to be addressed<br />
Question<br />
Introduction<br />
1<br />
2<br />
3<br />
4<br />
Are some women at greater<br />
risk of VTE?<br />
What is best practice for<br />
assessment of VTE risk?<br />
What is best practice for<br />
thromboprophylaxis for<br />
women with low, medium and<br />
high risk levels?<br />
What is best practice<br />
postnatal care and follow-up<br />
for a woman at risk of VTE?<br />
Likely Content/Headings/Document Flow<br />
Definition of VTE<br />
Incidence of VTE in <strong>Queensland</strong><br />
Clinical Standards<br />
Counselling of women<br />
Identify risk factors for VTE in pregnancy and the puerperium<br />
Identify a best practice method for VTE risk scoring<br />
Identify best practice methods of thromboprophylaxis<br />
Identify best practice in prophylactic management of VTE across risk levels<br />
and specific patient groups<br />
Identify best practice principles for discharge planning<br />
Identify teaching principles in relation to self-care in the puerperium<br />
including:<br />
<br />
<br />
Non-pharmacological VTE prevention methods<br />
Principles of self-management of pharmacological VTE prophylaxis,<br />
including :<br />
Warfarin<br />
Low molecular weight heparin<br />
Unfractionated heparin<br />
Identify best practice advice for follow-up e.g. self-referral to the GP, review<br />
by specialist<br />
Page 1 of 3
Translating evidence into best clinical practice<br />
Exclusions:<br />
Investigation and diagnosis of VTE in pregnancy and/or the puerperium<br />
Acute management of VTE occurring in pregnancy and/or the puerperium<br />
Table 3. Existing outcomes measures used in clinical audit for VTE<br />
VTE outcome measures<br />
Source<br />
Clinical audit standards*<br />
Australian<br />
Council of<br />
<strong>Health</strong>care<br />
Standards:<br />
Obstetric<br />
indicators<br />
Royal College of<br />
Obstetricians<br />
and<br />
Gynaecologists:<br />
Green-top<br />
Guideline 37a<br />
(2004)<br />
Indicator Area 6: Pharmacological thromboprophylaxis and caesarean section:<br />
CI. 6.1 Numerator: Total number of high risk women undergoing caesarean section who receive<br />
appropriate pharmacological thromboprophlylaxis<br />
Denominator: Total number of high risk women undergoing caesarean section<br />
Thromboprophylaxis during pregnancy, labour and after vaginal delivery<br />
Proportion of women with previous venous thromboembolism who undergo screening for<br />
thrombophilia<br />
Proportion of women with previous venous thromboembolism who receive six weeks postnatal<br />
Low Molecular Weight Heparin<br />
Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary<br />
embolism) in patients admitted to hospital<br />
National<br />
Institute for<br />
<strong>Health</strong> and<br />
Clinical<br />
Excellence:<br />
Guideline CG92,<br />
Audit support<br />
Selected items for ‘All patients:<br />
Criterion 1: Was the patient assessed for risk of VTE on admission?<br />
Criterion 2: Was the patient assessed for risk of bleeding before being offered<br />
pharmacological VTE prophylaxis?<br />
Criterion 3: Was the risks of bleeding and VTE reassessed within 24 hours of admission?<br />
Criterion 4: Was there evidence that the patient was encouraged to mobilise as soon as<br />
possible?<br />
Criterion 7: How long after the risk assessment was completed was pharmacological VTE<br />
prophylaxis started?<br />
Criterion 8: Was pharmacological VTE prophylaxis stopped when the patient was no longer at<br />
increased risk of VTE?<br />
Selected items for ‘Surgical patients’:<br />
Criterion 9, 11, 13, 17, 18: Does the patient fall into any of the following categories, if so, was<br />
the patient offered mechanical VTE prophylaxis?:<br />
Has had a surgical procedure with a total anaesthetic and surgical time of more than 90<br />
minutes, or 60 minutes if the surgery involves the pelvis or lower limb<br />
Expected significant reduction in mobility<br />
Acute surgical admission with inflammatory or intra-abdominal condition<br />
One or more of the following risk factors:<br />
Active cancer or cancer treatment<br />
Critical care admission<br />
Dehydration<br />
Known thrombophilias<br />
Obesity (BMI over 30 kg/m2)<br />
One or more significant medical co-morbidities<br />
Personal history or first-degree relative with a history of VTE<br />
Use of hormone replacement therapy<br />
Use of oestrogen-containing contraceptive therapy<br />
Varicose veins with phlebitis<br />
Page 2 of 3
Translating evidence into best clinical practice<br />
Table 3. Existing outcomes measures used in clinical audit for VTE continued<br />
VTE outcome measures<br />
Source<br />
Clinical audit standards*<br />
National<br />
Institute for<br />
<strong>Health</strong> and<br />
Clinical<br />
Excellence:<br />
Guideline CG92,<br />
Audit support<br />
Items for ‘All patients – provision of information<br />
Criterion 19: Before starting VTE prophylaxis was the patient offered verbal and written<br />
information about:<br />
The treatment and care they should be offered, [including being made aware of availability<br />
of written patient information available]?<br />
The risks and possible consequences of VTE?<br />
The importance of VTE prophylaxis and its possible side effects?<br />
The correct use of VTE prophylaxis (for example, anti-embolism stockings, foot impulse or<br />
intermittent pneumatic compression devices)?<br />
How patients can reduce their risk of VTE (such as keeping well hydrated and, if possible,<br />
exercising and becoming more mobile)?<br />
Criterion 20: As part of the discharge plan, was the patient offered verbal and written<br />
information on:<br />
The signs and symptoms of deep vein thrombosis and pulmonary embolism?<br />
The correct and recommended duration of use of VTE prophylaxis at home (if discharged<br />
with prophylaxis)?<br />
The importance of using VTE prophylaxis correctly and continuing treatment for the<br />
recommended duration (if discharged with prophylaxis)?<br />
The signs and symptoms of adverse events related to VTE prophylaxis (if discharged with<br />
prophylaxis)?<br />
The importance of seeking help and who to contact if they have any problems using the<br />
prophylaxis?<br />
The importance of seeking medical help if deep vein thrombosis, pulmonary embolism or<br />
another adverse event is suspected?<br />
*For source documents refer to:<br />
ACHS Obstetric Manual: http://www.anzca.edu.au/resources/college-publications/books-andpublications/ClinicalIndicators.pdf<br />
Royal College of Obstetricians and Gynaecologists Guideline No. 37a (2004) Thromboprophylaxis during<br />
pregnancy, labour and after vaginal delivery: http:// www.rcog.org.uk/guidelines:<br />
National Institute for <strong>Health</strong> and Clinical Excellence Guideline CG92, Audit support:<br />
http://www.nice.org.uk/nicemedia/live/12695/47202/47202.doc<br />
Page 3 of 3