PROMUS Element⢠Plus - Boston Scientific
PROMUS Element⢠Plus - Boston Scientific
PROMUS Element⢠Plus - Boston Scientific
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Table 10.1.8. PLaTinUM workhorse 12-Month Clinical Endpoints by gender, intent-to-Treat,<br />
PrOMUS Element Male and female Patients (n=768)<br />
PrOMUS Element Stent<br />
Male Patients<br />
(n=550)<br />
PrOMUS Element Stent<br />
female Patients<br />
(n=218)<br />
Efficacy<br />
TVR, Overall 2.8% (15/532) 2.3% (5/213)<br />
TLR, Overall 1.7% (9/532) 2.3% (5/213)<br />
TLR, PCI 1.3% (7/532) 1.4% (3/213)<br />
TLR, CABG 0.4% (2/532) 0.9% (2/213)<br />
Non-TLR, Overall 1.3% (7/532) 0.0% (0/213)<br />
Non-TLR, PCI 1.1% (6/532) 0.0% (0/213)<br />
Non-TLR, CABG 0.2% (1/532) 0.0% (0/213)<br />
TLF<br />
SafETY<br />
3.4% (18/529) 3.8% (8/213)<br />
Total Death 1.7% (9/532) 0.5% (1/213)<br />
Cardiac Death or MI 2.3% (12/532) 1.4% (3/213)<br />
Cardiac Death 1.1% (6/532) 0.5% (1/213)<br />
MI 1.1% (6/532) 0.9% (2/213)<br />
Q-wave MI 0.2% (1/532) 0.0% (0/213)<br />
Non-Q-wave MI<br />
ARC Stent Thrombosis<br />
0.9% (5/532) 0.9% (2/213)<br />
Definite or Probable 0.6% (3/524) 0.0% (0/211)<br />
Definite 0.6% (3/524) 0.0% (0/211)<br />
Probable 0.0% (0/524) 0.0% (0/211)<br />
This trial was not sized to determine the rate of low frequency events with a pre-specified<br />
precision.<br />
Numbers are % (count/sample size).<br />
Abbreviations: ARC=Academic Research Consortium; CABG=coronary artery bypass<br />
grafting; MI=myocardial infarction; PCI=percutaneous coronary intervention; TLF=target<br />
lesion failure; TLR=target lesion revascularization; TVR=target vessel revascularization.<br />
Figures 10.1.2 and 10.1.3 show the cumulative rate of TLF through 12-months for males and females,<br />
respectively. This post hoc analysis shows that there were similar TLF rates for <strong>PROMUS</strong> Element<br />
and <strong>PROMUS</strong>® groups for males and females at all follow-up time-points (30 days, 6 months,<br />
and 12 months).<br />
figure 10.1.2. PLaTinUM workhorse Cumulative rate of Target Lesion failure to 12 Months,<br />
intent-to-Treat, Event rate ± 1.5 SE, all Male Patients, (n=1092)<br />
Event rate Event free<br />
PrOMUS Element 3.4% 96.6%<br />
PrOMUS DES Control 3.1% 96.9%<br />
13<br />
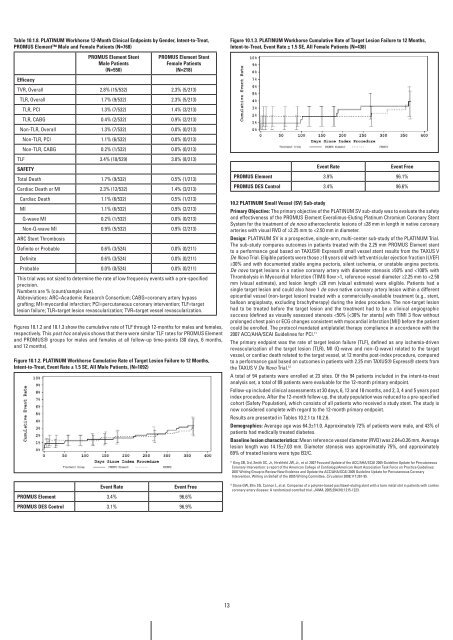
figure 10.1.3. PLaTinUM workhorse Cumulative rate of Target Lesion failure to 12 Months,<br />
intent-to-Treat, Event rate ± 1.5 SE, all female Patients (n=438)<br />
Event rate Event free<br />
PrOMUS Element 3.9% 96.1%<br />
PrOMUS DES Control 3.4% 96.6%<br />
10.2 PLaTinUM Small vessel (Sv) Sub-study<br />
Primary Objective: The primary objective of the PLATINUM SV sub-study was to evaluate the safety<br />
and effectiveness of the <strong>PROMUS</strong> Element Everolimus-Eluting Platinum Chromium Coronary Stent<br />
System for the treatment of de novo atherosclerotic lesions of ≤28 mm in length in native coronary<br />
arteries with visual RVD of ≥2.25 mm to