PROMUS Element⢠Plus - Boston Scientific
PROMUS Element⢠Plus - Boston Scientific
PROMUS Element⢠Plus - Boston Scientific
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Demographics: Average age was 61.8±9.9. 77% of patients were male, and 19% of patients had<br />
medically treated diabetes.<br />
baseline lesion characteristics: Reference vessel diameter was 2.72±0.53 mm with baseline lesion<br />
length 15.40±7.03 mm. Percent diameter stenosis was 74.09±10.93 and 67% of treated lesions were<br />
type B2/C.<br />
12-Month Clinical Outcomes<br />
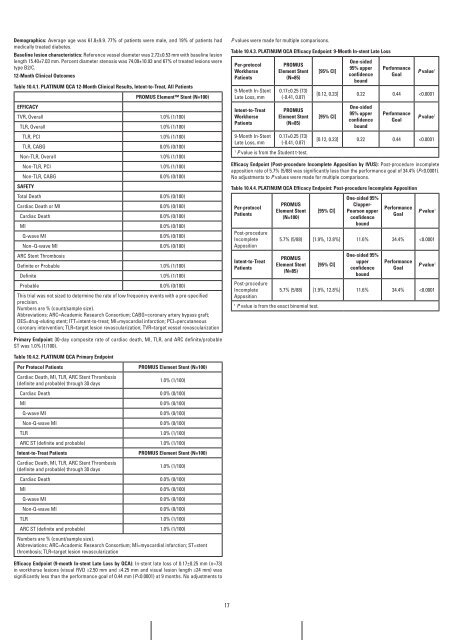
Table 10.4.1. PLaTinUM QCa 12-Month Clinical results, intent-to-Treat, all Patients<br />
PrOMUS Element Stent (n=100)<br />
EffiCaCY<br />
TVR, Overall 1.0% (1/100)<br />
TLR, Overall 1.0% (1/100)<br />
TLR, PCI 1.0% (1/100)<br />
TLR, CABG 0.0% (0/100)<br />
Non-TLR, Overall 1.0% (1/100)<br />
Non-TLR, PCI 1.0% (1/100)<br />
Non-TLR, CABG 0.0% (0/100)<br />
SafETY<br />
Total Death 0.0% (0/100)<br />
Cardiac Death or MI 0.0% (0/100)<br />
Cardiac Death 0.0% (0/100)<br />
MI 0.0% (0/100)<br />
Q-wave MI 0.0% (0/100)<br />
Non−Q-wave MI 0.0% (0/100)<br />
ARC Stent Thrombosis<br />
Definite or Probable 1.0% (1/100)<br />
Definite 1.0% (1/100)<br />
Probable 0.0% (0/100)<br />
This trial was not sized to determine the rate of low frequency events with a pre-specified<br />
precision.<br />
Numbers are % (count/sample size).<br />
Abbreviations: ARC=Academic Research Consortium; CABG=coronary artery bypass graft;<br />
DES=drug-eluting stent; ITT=intent-to-treat; MI=myocardial infarction; PCI=percutaneous<br />
coronary intervention; TLR=target lesion revascularization; TVR=target vessel revascularization<br />
Primary Endpoint: 30-day composite rate of cardiac death, MI, TLR, and ARC definite/probable<br />
ST was 1.0% (1/100).<br />
Table 10.4.2. PLaTinUM QCa Primary Endpoint<br />
Per Protocol Patients PrOMUS Element Stent (n=100)<br />
Cardiac Death, MI, TLR, ARC Stent Thrombosis<br />
1.0% (1/100)<br />
(definite and probable) through 30 days<br />
Cardiac Death 0.0% (0/100)<br />
MI 0.0% (0/100)<br />
Q-wave MI 0.0% (0/100)<br />
Non-Q-wave MI 0.0% (0/100)<br />
TLR 1.0% (1/100)<br />
ARC ST (definite and probable) 1.0% (1/100)<br />
intent-to-Treat Patients PrOMUS Element Stent (n=100)<br />
Cardiac Death, MI, TLR, ARC Stent Thrombosis<br />
(definite and probable) through 30 days<br />
1.0% (1/100)<br />
Cardiac Death 0.0% (0/100)<br />
MI 0.0% (0/100)<br />
Q-wave MI 0.0% (0/100)<br />
Non-Q-wave MI 0.0% (0/100)<br />
TLR 1.0% (1/100)<br />
ARC ST (definite and probable) 1.0% (1/100)<br />
Numbers are % (count/sample size).<br />
Abbreviations: ARC=Academic Research Consortium; MI=myocardial infarction; ST=stent<br />
thrombosis; TLR=target lesion revascularization<br />
Efficacy Endpoint (9-month in-stent Late Loss by QCa): In-stent late loss of 0.17±0.25 mm (n=73)<br />
in workhorse lesions (visual RVD ≥2.50 mm and ≤4.25 mm and visual lesion length ≤24 mm) was<br />
significantly less than the performance goal of 0.44 mm (P