Electrochemistry
Electrochemistry
Electrochemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
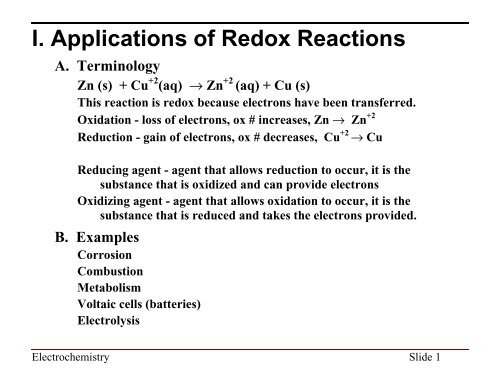
I. Applications of Redox Reactions<br />
A. Terminology<br />
Zn (s) + Cu +2 (aq) fi Zn +2 (aq) + Cu (s)<br />
This reaction is redox because electrons have been transferred.<br />
Oxidation - loss of electrons, ox # increases, Zn fi Zn +2<br />
Reduction - gain of electrons, ox # decreases, Cu +2 fi Cu<br />
Reducing agent - agent that allows reduction to occur, it is the<br />
substance that is oxidized and can provide electrons<br />
Oxidizing agent - agent that allows oxidation to occur, it is the<br />
substance that is reduced and takes the electrons provided.<br />
B. Examples<br />
Corrosion<br />
Combustion<br />
Metabolism<br />
Voltaic cells (batteries)<br />
Electrolysis<br />
<strong>Electrochemistry</strong> Slide 1