Electrochemistry
Electrochemistry
Electrochemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
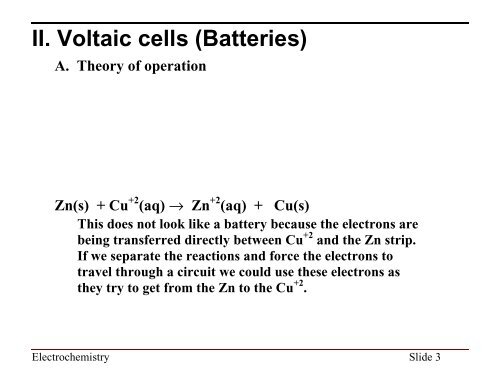
II. Voltaic cells (Batteries)<br />
A. Theory of operation<br />
Zn(s) + Cu +2 (aq) fi Zn +2 (aq) + Cu(s)<br />
This does not look like a battery because the electrons are<br />
being transferred directly between Cu +2 and the Zn strip.<br />
If we separate the reactions and force the electrons to<br />
travel through a circuit we could use these electrons as<br />
they try to get from the Zn to the Cu +2 .<br />
<strong>Electrochemistry</strong> Slide 3