Book of abstracts - State Scientific Institution “Institute for Single ...
Book of abstracts - State Scientific Institution “Institute for Single ...
Book of abstracts - State Scientific Institution “Institute for Single ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Abstracts Poster – Part III: Probes, Labels and Sensors<br />
PRLS-44<br />
Optochemical oxygen sensing using Pt(II)-porphyrin dye immobilised on<br />
S-layer matrices<br />
Sylvia Scheicher 1 , Stefan Köstler 1,3 , Birgit Kainz 2 , Christian Konrad 3 , Michael Suppan 3 ,<br />
Alessandro Bizzari 3 , Dietmar Pum 2 and Volker Ribitsch 1,3<br />
1) Karl Franzens University Graz, Institute <strong>of</strong> Physical Chemistry, A-8010 (Austria).<br />
2) University <strong>of</strong> Natural Resources and Applied Life Sciences, Centre <strong>of</strong> Nanobiotechnology,<br />
A-1180 Wien (Austria)<br />
3) Joanneum Research, Institute <strong>of</strong> Chemical Process Development and Control, A-8010 Graz (Austria)<br />
Crystalline bacterial surface layers (S-layers) appeared to be perfect matrices <strong>for</strong> immobilizing functional<br />
molecules in a highly ordered structure. S-layers are monomolecular arrays <strong>of</strong> a single type <strong>of</strong> protein and<br />
exhibit different types <strong>of</strong> lattice symmetry depending on the protein structure. The capability <strong>of</strong> S-layer<br />
proteins to reassemble in suspension, on solid surfaces and liquid films makes them an ideal substrate <strong>for</strong><br />
immobilising (macro) molecules. [1-2] The protein SbpA, used in this study, <strong>for</strong>ms an S-layer lattice <strong>of</strong><br />
square symmetry with a center-to-center spacing <strong>of</strong> 13.1 nm. Each morphological unit is composed <strong>of</strong> four<br />
identical subunits.<br />
Pt(II) complexes <strong>of</strong> porphyrins show strong phosphorescence at room temperature with decay times in the<br />
range <strong>of</strong> several tens <strong>of</strong> µs. The excited triplet states <strong>of</strong> these dyes can be quenched by molecular oxygen.<br />
This leads to a marked decrease <strong>of</strong> luminescence lifetime and intensity and can be exploited <strong>for</strong> optical<br />
oxygen sensing. [3-4]<br />
S-layer protein SbpA was recrystallised onto glass substrates. The free carboxylic groups <strong>of</strong> the protein<br />
were used to covalently bind the sensitive dye via the <strong>for</strong>mation <strong>of</strong> active ester intermediates. 5,10,15,20-<br />
Tetrakis-(4-aminophenyl)-porphyrin-Pt-(II) was subsequently immobilized to the protein layer.<br />
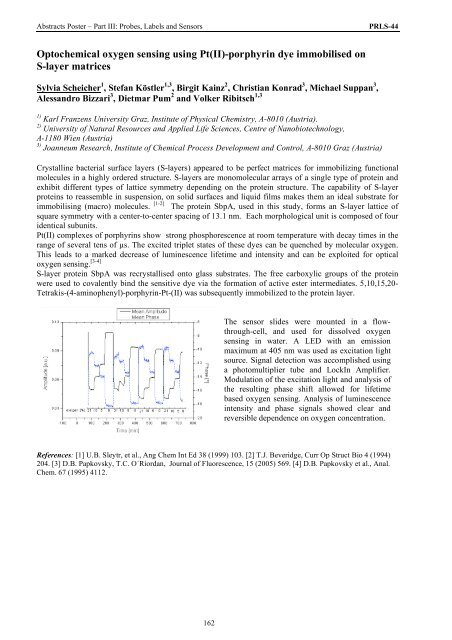
The sensor slides were mounted in a flowthrough-cell,<br />
and used <strong>for</strong> dissolved oxygen<br />
sensing in water. A LED with an emission<br />
maximum at 405 nm was used as excitation light<br />
source. Signal detection was accomplished using<br />
a photomultiplier tube and LockIn Amplifier.<br />
Modulation <strong>of</strong> the excitation light and analysis <strong>of</strong><br />
the resulting phase shift allowed <strong>for</strong> lifetime<br />
based oxygen sensing. Analysis <strong>of</strong> luminescence<br />
intensity and phase signals showed clear and<br />
reversible dependence on oxygen concentration.<br />
References: [1] U.B. Sleytr, et al., Ang Chem Int Ed 38 (1999) 103. [2] T.J. Beveridge, Curr Op Struct Bio 4 (1994)<br />
204. [3] D.B. Papkovsky, T.C. O´Riordan, Journal <strong>of</strong> Fluorescence, 15 (2005) 569. [4] D.B. Papkovsky et al., Anal.<br />
Chem. 67 (1995) 4112.<br />
162







![Changes in crystalli] ation conditions when growing large single ...](https://img.yumpu.com/23526254/1/184x260/changes-in-crystalli-ation-conditions-when-growing-large-single-.jpg?quality=85)






