School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
School of Engineering and Science - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
group <strong>of</strong> compounds becomes gradually more <strong>and</strong> more diffuse. Many reports,[19] books<br />
[4] <strong>and</strong> reviews [2, 20] have been published on this topic, showing an enormous molecular<br />
diversity amongst the inorganic family <strong>of</strong> molecules. This diversity is a consequence <strong>of</strong><br />
the rich, unprecedented <strong>and</strong> unusual properties associated to POMs. Many authors claim<br />
that they can be regarded as packed arrays <strong>of</strong> pyramidal MO 5 <strong>and</strong> octahedral MO 6 units.<br />
These entities are then the fundamental structural units <strong>and</strong> are somewhat similar to the<br />
-CH 2 - unit in organic molecules. The very important MO 6 units (<strong>and</strong> the MO 5 partner<br />
as well but to a lesser extent) are packed to form countless shapes. They join to one<br />
another apparently, in accordance with a few simple rules (as -CH 2 - does in organic molecules).<br />
Observing a representative set <strong>of</strong> POM clusters <strong>and</strong> identifying the MO 6 blocks,<br />
one can notice that the molecule as a whole is built by edge- <strong>and</strong>/or corner-sharing MO 6<br />
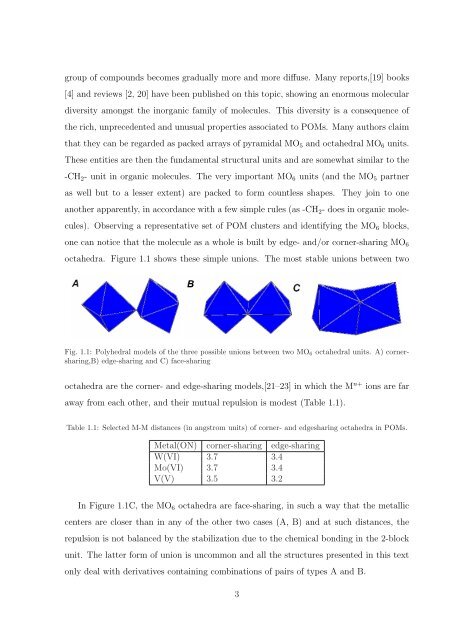
octahedra. Figure 1.1 shows these simple unions. The most stable unions between two<br />
Fig. 1.1: Polyhedral models <strong>of</strong> the three possible unions between two MO 6 octahedral units. A) cornersharing,B)<br />
edge-sharing <strong>and</strong> C) face-sharing<br />
octahedra are the corner- <strong>and</strong> edge-sharing models,[21–23] in which the M n+ ions are far<br />
away from each other, <strong>and</strong> their mutual repulsion is modest (Table 1.1).<br />
Table 1.1: Selected M-M distances (in angstrom units) <strong>of</strong> corner- <strong>and</strong> edgesharing octahedra in POMs.<br />
Metal(ON) corner-sharing edge-sharing<br />
W(VI) 3.7 3.4<br />
Mo(VI) 3.7 3.4<br />
V(V) 3.5 3.2<br />
In Figure 1.1C, the MO 6 octahedra are face-sharing, in such a way that the metallic<br />
centers are closer than in any <strong>of</strong> the other two cases (A, B) <strong>and</strong> at such distances, the<br />
repulsion is not balanced by the stabilization due to the chemical bonding in the 2-block<br />
unit. The latter form <strong>of</strong> union is uncommon <strong>and</strong> all the structures presented in this text<br />
only deal with derivatives containing combinations <strong>of</strong> pairs <strong>of</strong> types A <strong>and</strong> B.<br />
3