+ - E
+ - E
+ - E
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
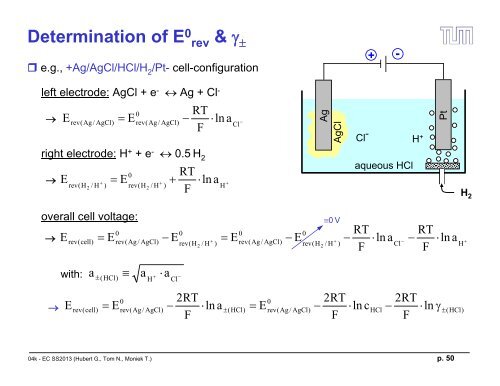
Determination of E 0 rev & <br />
e.g., +Ag/AgCl/HCl/H 2<br />
/Pt- cell-configuration<br />
+ -<br />
left electrode: AgCl + e - Ag + Cl -<br />
<br />
E<br />
rev(Ag/<br />
AgCl)<br />
<br />
E<br />
0<br />
rev(Ag/ AgCl)<br />
<br />
RT<br />
F<br />
ln<br />
a<br />
Cl<br />
<br />
Ag<br />
AgCl<br />
Cl - H +<br />
Pt<br />
right electrode: H + + e - 0.5 H 2<br />
0 RT<br />
E<br />
E<br />
ln<br />
a<br />
rev(H2<br />
/ H ) rev(H2<br />
/ H )<br />
F<br />
H<br />
<br />
aqueous HCl<br />
H 2<br />
overall cell voltage:<br />
<br />
E<br />
rev(cell)<br />
E<br />
0<br />
rev(Ag/ AgCl)<br />
E<br />
0<br />
rev(H<br />
2<br />
/ H<br />
<br />
)<br />
<br />
E<br />
0<br />
rev(Ag/ AgCl)<br />
E<br />
0<br />
rev(H<br />
2<br />
0V<br />
/ H<br />
<br />
)<br />
<br />
RT<br />
F<br />
ln a<br />
Cl<br />
<br />
<br />
RT<br />
F<br />
ln<br />
a<br />
H<br />
<br />
with:<br />
a<br />
<br />
a a<br />
( HCl)<br />
H<br />
Cl<br />
<br />
<br />
E<br />
rev(cell)<br />
E<br />
0<br />
rev(Ag/ AgCl)<br />
<br />
2RT<br />
F<br />
ln a<br />
(HCl)<br />
<br />
E<br />
0<br />
rev(Ag/ AgCl)<br />
<br />
2RT<br />
F<br />
ln c<br />
HCl<br />
<br />
2RT<br />
F<br />
ln<br />
<br />
(HCl)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 50
Determination of E 0 rev & <br />
e.g., +Ag/AgCl/HCl/H 2<br />
/Pt- cell-configuration<br />
+ -<br />
a) E 0 rev(Ag/AgCl) :<br />
Ag<br />
AgCl<br />
Cl - H +<br />
Pt<br />
E<br />
2RT<br />
ln c<br />
F<br />
E<br />
2RT<br />
ln<br />
<br />
F<br />
0<br />
<br />
rev(cell)<br />
HCl rev(Ag/ AgCl)<br />
(HCl)<br />
aqueous HCl<br />
H 2<br />
considering that:<br />
(see pg. 48)<br />
ln<br />
<br />
I c<br />
( HCl)<br />
HCl<br />
determination of E 0 rev(Ag/Ag/Cl)<br />
b) (HCl)<br />
:<br />
ln<br />
F<br />
<br />
2RT<br />
0<br />
E<br />
rev(Ag/ AgCl)<br />
Erev(cell)<br />
<br />
ln<br />
HCl<br />
( HCl)<br />
c<br />
<br />
from: C.H. Hamann & W. Vielstich; Electrochemie; Wiley-VCH (2005)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 51
Diffusion Potentials<br />
+ -<br />
E<br />
rev(left)<br />
<br />
E<br />
0<br />
rev(left)<br />
0.5 H 2<br />
H + + e - right<br />
<br />
RT<br />
F<br />
ln<br />
<br />
a<br />
H<br />
<br />
left<br />
e - H +<br />
0.5 H 2<br />
H + + e -<br />
metal<br />
HCl (0.1M)<br />
HCl (0.01M)<br />
H +<br />
metal<br />
E<br />
rev(right)<br />
<br />
E<br />
0<br />
rev(right)<br />
<br />
RT<br />
F<br />
ln<br />
a<br />
H<br />
<br />
H 2 H 2<br />
<br />
E<br />
cell<br />
<br />
E<br />
rev(left)<br />
E<br />
rev(right)<br />
<br />
E<br />
0<br />
rev(left)<br />
E<br />
0<br />
rev(right)<br />
<br />
RT<br />
F<br />
<br />
ln<br />
<br />
<br />
<br />
<br />
a<br />
a<br />
H<br />
H<br />
<br />
<br />
<br />
<br />
left<br />
right<br />
<br />
<br />
<br />
<br />
RT<br />
F<br />
<br />
ln<br />
<br />
<br />
<br />
<br />
a<br />
a<br />
H<br />
H<br />
<br />
<br />
<br />
<br />
left<br />
right<br />
<br />
0<br />
<br />
<br />
left: H + + e - 0.5 H 2<br />
cathodic reaction<br />
decrease of c HCl <br />
Cl - move to the right<br />
Cl - flux t Cl-<br />
H + flux t H+<br />
+ -<br />
right: 0.5 H 2<br />
H + + e -<br />
anodic reaction<br />
increase of c HCl <br />
H + move to the left<br />
<br />
E<br />
cell<br />
E<br />
rev(left)<br />
E<br />
rev(right)<br />
E<br />
Nernst<br />
E junction<br />
RT a<br />
<br />
H<br />
E<br />
junction<br />
ln<br />
F a<br />
<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 52<br />
<br />
H<br />
left<br />
right<br />
<br />
<br />
<br />
<br />
<br />
<br />
left<br />
<br />
right
Diffusion Potentials<br />
derivation of E junction<br />
lengthy, but in the simplest case for a 1:1 electrolyte:<br />
E<br />
junction<br />
<br />
<br />
<br />
t<br />
t <br />
left<br />
right<br />
<br />
<br />
ln<br />
<br />
a<br />
<br />
<br />
left<br />
a<br />
right <br />
if t +<br />
t -<br />
E junction<br />
0V (e.g.: KCl: t + = 0.49; KNO 3 : t + = 0.51)<br />
more precise evaluation: Henderson equation (s. chapter 3.2.4 in Hamann & Vielstich)<br />
<br />
<br />
RT<br />
F<br />
use of a salt-bridge with concentrated KCl or KNO 3<br />
minimizes E junction<br />
from: C.H. Hamann, A. Hamnett, W. Vielstich; Electrochemistry; Wiley-VCH (2007)<br />
from: A.J. Bard & L.R. Faulkner;<br />
Electrochemical Methods; Wiley (1980)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 53
electrochemical cells, thermodynamics, reference electrodes<br />
ionic conduction in electrolytes / activity coefficients<br />
electrochemical kinetics<br />
mass transport / diffusion effects<br />
cyclic voltammetry / rotating disk electrodes / -electrodes<br />
AC impedance<br />
thermoelectrica and solid electrolytes<br />
spectroscopic methods<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 54
Electrocatalysis<br />
heterogeneously catalyzed electrochemical reactions are a complex sequence of possibly<br />
many steps: adsorption of reactants, desorption of products, solvation, etc.<br />
in this sequence of processes, the rate-determining steps (rds) may be different on<br />
different electrocatalyts<br />
o<br />
O + ne - R<br />
R ; E rev(O/R)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 55
Overpotentials<br />
overpotentials: deviation from E rev<br />
as current is passed through the electrode<br />
activation overpotential, act<br />
, is the overpotential in absence<br />
Cl H 2<br />
2H + + 2e -<br />
2<br />
+ 2e - 2Cl -<br />
of ohmic losses and conc. gradients<br />
metal<br />
metal<br />
E cell<br />
aqueous HCl<br />
Cl 2 H 2<br />
Cl -<br />
<br />
act<br />
where:<br />
E E<br />
electrode<br />
rev<br />
act<br />
0 for anodic process<br />
act<br />
0 for cathodic process<br />
from: C.H. Hamann, A. Hamnett, W. Vielstich; Electrochemistry; Wiley-VCH (2007)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 56
Driving Force for Electron Transfer<br />
consider activated transition state: A + B (AB) <br />
<br />
r<br />
f<br />
<br />
k<br />
0<br />
f<br />
c<br />
A<br />
c<br />
B<br />
<br />
exp<br />
<br />
<br />
G<br />
f<br />
RT<br />
<br />
<br />
<br />
cathodic (reduction) reaction:<br />
ox + ne - (Me) red<br />
anodic (oxidation) direction:<br />
red ox + ne - (Me)<br />
potential getting more negative <br />
= 2<br />
– 1<br />
Electrokinetics<br />
the Butler-Volmer equation is generally used to describe the overpotential, temperature,<br />
and reactant concentration dependence of the current of an electrode reaction *) :<br />
based on Hamann & Vielstich,<br />
Electrochemie, Wiley (2005):<br />
with:<br />
o<br />
O + ne - R<br />
R ; E rev(O/R)<br />
nF<br />
<br />
<br />
RT<br />
i i0(T,c<br />
,c )<br />
rf e e<br />
O R<br />
<br />
<br />
(1<br />
) nF<br />
<br />
RT<br />
i anodic (>0) i cathodic (
Variants of the Butler-Volmer Equation<br />
often the Butler-Volmer equation is written as log 10 & with anodic/cathodic transfer coeff.:<br />
i<br />
i<br />
0( T , c<br />
O<br />
, c<br />
R<br />
)<br />
rf<br />
<br />
<br />
<br />
<br />
a<br />
F<br />
c<br />
F<br />
<br />
<br />
<br />
2.303<br />
RT<br />
2.303<br />
RT<br />
10 10 i0(<br />
T , c<br />
<br />
O , cR<br />
)<br />
<br />
rf<br />
<br />
<br />
<br />
<br />
10<br />
<br />
b<br />
a<br />
<br />
10<br />
<br />
b<br />
c<br />
<br />
<br />
<br />
<br />
where:<br />
b<br />
a,c<br />
<br />
2.303<br />
RT<br />
F<br />
a,<br />
c<br />
is referred to as the anodic/cathodic Tafel slope, representing the<br />
overpotential increase required for a 10x increase in current<br />
at 25°C, b commonly ranges from 120 mV/decade ( a,c =0.5)<br />
to 30 mV/decade ( a,c = 2)<br />
nF<br />
(1<br />
) nF<br />
<br />
special case:<br />
RT<br />
RT<br />
i i<br />
<br />
0(T,c<br />
,c )<br />
rf<br />
e e<br />
O R<br />
<br />
<br />
<br />
<br />
here, is also referred to as transfer coefficient, even though its meaning is<br />
different from the definitions in [2] and [3] (actually, in [1], the symmetry factor is<br />
meant! ...detailed explanation of differences: E. Gileadi, Electrode Kinetics...VCH)<br />
care must be taken to not mix up these two different definitions<br />
the more general definition with a,c as transfer coefficients will be used here<br />
[1] A.J. Bard & L.R. Faulkner, Electrochemical Methods, John Wiley & Sons (1980); [2] J. O’M. Bockris<br />
& A.K.N. Reddy, Modern Electrochemistry, Plenum Press: (1970); [3] J.S. Newman, Electrochemical Systems, Prentice Hall (1991).<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 59
Dependence of i 0 on the Electrocatalyst<br />
exchange current densities vary by many order of magnitudes for different electrocatalysts<br />
e.g., for the H 2 evolution reaction (2H + +2e - H 2 ) in acid electrolytes:<br />
Pt<br />
Rh<br />
Re<br />
Au<br />
Ir<br />
Cu<br />
Ni<br />
Co<br />
Fe<br />
W<br />
Pb<br />
Tl<br />
Sn<br />
Zn<br />
Cd<br />
Bi Ag<br />
Ga<br />
Mo<br />
Nb<br />
Ti Ta<br />
from: S. Trasatti, J. Electroanal.<br />
Chem. 39 (1972) 163<br />
according to Sabatier’s Principle, high reaction rates (i 0 ’s) require bonding of<br />
the reaction intermediate (M-H) which is not too weak and not too strong<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 60
Temperature and Concentration Dependence of i 0<br />
as any rate constant of a chemical reaction, the exchange current density depends<br />
on temperature and reactant/products concentrations<br />
it is frequently based on the definition used in [3]:<br />
i<br />
i<br />
0 ( T , cO<br />
, cR<br />
)<br />
* * *<br />
0( T , cO<br />
, cR<br />
)<br />
<br />
<br />
<br />
<br />
c<br />
c<br />
R<br />
*<br />
R<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
c<br />
c<br />
O<br />
*<br />
O<br />
<br />
<br />
<br />
<br />
e<br />
E<br />
RT<br />
act<br />
with:<br />
<br />
i<br />
0( T<br />
*<br />
*<br />
*<br />
, c O , c R )<br />
[A/cm 2 real]: exchange current density at defined reference temperature and<br />
reference reactant/product (c * R , c * O ) concentrations<br />
, [dimensionless]: reaction orders, describing the concentration dependence of i 0<br />
E act [J/mol]: activation energy of the exchange current density<br />
[1] A.J. Bard & L.R. Faulkner, Electrochemical Methods, John Wiley & Sons (1980); [2] J. O’M. Bockris<br />
& A.K.N. Reddy, Modern Electrochemistry, Plenum Press: (1970); [3] J.S. Newman, Electrochemical Systems, Prentice Hall (1991).<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 61
Characteristics of the Butler-Volmer Reaction<br />
the Butler-Volmer equation is the summation of anodic and cathodic currents; this is shown<br />
for the example of the HOR/HER kinetics on low-index Pt single crystals:<br />
HOR/HER on Pt(hkl) (0.05M H 2 SO 4 at 60°C)<br />
Pt face (110) (100) (111)<br />
i 0 [mA/cm 2 ] 1.35 0.76 0.83<br />
b [mV/decade] 33 44 66<br />
(Markovic et al., J. Phys. Chem. B 101 (1997) 5405))<br />
where rf = 1 for flame-annealed Pt(hkl) single crystals<br />
i<br />
net<br />
i<br />
0( T , c<br />
O<br />
, c<br />
R<br />
)<br />
rf<br />
<br />
<br />
10<br />
<br />
<br />
<br />
b<br />
a<br />
i anodic<br />
<br />
10<br />
<br />
b<br />
c<br />
i cathodic<br />
at =0: i anodic<br />
= i cathodic<br />
=i 0(T,H2, H+)<br />
dynamic equilibrium<br />
<br />
<br />
<br />
<br />
2<br />
Pt ]<br />
current density [ mA/cm<br />
8<br />
6<br />
4<br />
2<br />
0<br />
-2<br />
-4<br />
-6<br />
i anodic : H 2 2H + + 2e - i net<br />
(i 0 rf )<br />
i cathodic : 2H + + 2e - H 2<br />
at +b a<br />
/2: i anodic<br />
10 i cathodic<br />
reverse reaction negligible<br />
-8<br />
-40 -30 -20 -10 0 10 20 30 40<br />
overpotential [mV]<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 62
2<br />
Tafel Slope Impact – HOR/HER Example<br />
for the HOR/HER on low-index Pt single crystal faces, nearly identical i 0<br />
’s were<br />
reported, while the Tafel slopes varied from 33 66 mV/dec:<br />
Pt ]<br />
current density [ mA/cm<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
-5<br />
-10<br />
-15<br />
-20<br />
-25<br />
HOR/HER on Pt(hkl) (0.05M H 2 SO 4 at 60°C)<br />
Pt face (110) (100) (111)<br />
i 0 [mA/cm 2 ] 1.35 0.76 0.83<br />
b [mV/decade] 33 44 66<br />
(Markovic et al., J. Phys. Chem. B 101 (1997) 5405))<br />
2H + + 2e - H 2<br />
Pt(111)<br />
Pt(100)<br />
Pt(110)<br />
Pt(110)<br />
-100 -80 -60 -40 -20 0 20 40 60 80 100<br />
overpotential [mV]<br />
Pt(100)<br />
Pt(111)<br />
H 2 2H + + 2e -<br />
from the definition of b:<br />
at 60ºC (333 K) and b HOR/HER<br />
from table<br />
2.303RT<br />
<br />
0.033V<br />
F<br />
HOR , HER<br />
<br />
2.303RT<br />
<br />
0.066V<br />
F<br />
HOR , HER<br />
<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 63<br />
<br />
<br />
the overpotential effect on current density<br />
is the stronger, the lower the Tafel slope<br />
near =0, i<br />
2<br />
1
Butler-Volmer: Linear Approximation<br />
in the region of small overpotentials, the Butler-Volmer equation can be linearized:<br />
for:<br />
a<br />
F<br />
RT<br />
c<br />
F<br />
<br />
1 and <br />
1<br />
RT<br />
i.e.,<br />
RT<br />
<br />
F<br />
a<br />
and<br />
RT<br />
<br />
F<br />
b<br />
<br />
e<br />
a<br />
F<br />
<br />
RT<br />
a<br />
F<br />
1<br />
RT<br />
<br />
and<br />
e<br />
c<br />
F<br />
<br />
RT<br />
1<br />
c<br />
F<br />
RT<br />
<br />
therefore:<br />
i i0(<br />
T , c , c )<br />
O<br />
R<br />
rf<br />
<br />
<br />
<br />
<br />
e<br />
a<br />
F<br />
<br />
RT<br />
<br />
e<br />
c<br />
F<br />
<br />
RT<br />
<br />
<br />
<br />
<br />
<br />
i i<br />
0( T , c<br />
O<br />
, c<br />
R<br />
)<br />
rf<br />
( a<br />
<br />
<br />
) F<br />
c<br />
RT<br />
<br />
<br />
i<br />
0( T , c<br />
O<br />
, c<br />
R<br />
)<br />
rf<br />
2.303<br />
<br />
<br />
<br />
1<br />
b<br />
a<br />
<br />
1<br />
b<br />
c<br />
<br />
<br />
<br />
<br />
special case as defined by Bard and Faulkner:<br />
(1<br />
) n<br />
a<br />
i<br />
i<br />
and<br />
0( T , c O , c )<br />
R<br />
rf<br />
<br />
n<br />
c<br />
F n<br />
<br />
RT<br />
<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 64
Butler-Volmer: Linear Approximation<br />
for the previous example of HOR/HER on Pt(110):<br />
2<br />
Pt ]<br />
current density [ mA/cm<br />
6<br />
4<br />
2<br />
0<br />
-2<br />
-4<br />
-6<br />
HOR/HER on Pt(hkl) (0.05M H 2 SO 4 at 60°C)<br />
Pt face (110) (100) (111)<br />
i 0 [mA/cm 2 ] 1.35 0.76 0.83<br />
b [mV/decade] 33 44 66<br />
(Markovic et al., J. Phys. Chem. B 101 (1997) 5405))<br />
< 10% error at - < b /3<br />
< 10% error at < b /3<br />
-40 -30 -20 -10 0 10 20 30 40<br />
overpotential [mV]<br />
since:<br />
<br />
R<br />
ct<br />
i<br />
0(T,cO<br />
,cR<br />
)<br />
<br />
<br />
i<br />
rf<br />
(<br />
<br />
R T<br />
<br />
F i<br />
0(T,c<br />
<br />
O<br />
,c<br />
R<br />
)<br />
1<br />
rf<br />
(<br />
<br />
a<br />
<br />
obtain i 0 from the slope in the<br />
linear region, if a,c & rf are known<br />
rf from cyclic voltammetry,<br />
XRD, or TEM<br />
a,c from Tafel plots<br />
if simpler Eqn. on pg. 58 applies:<br />
a + c = + (1-) = 1<br />
<br />
R<br />
ct<br />
<br />
<br />
i<br />
a<br />
R T<br />
<br />
F i<br />
0(T,c<br />
c<br />
O<br />
R T<br />
) <br />
F<br />
1<br />
,c<br />
R<br />
)<br />
rf<br />
i<br />
<br />
c<br />
)<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 65
Butler-Volmer: Tafel Approximation<br />
at large overpotentials, one of the Butler-Volmer equation terms becomes negligible:<br />
for:<br />
<br />
<br />
1 and 1<br />
b a<br />
b c<br />
i.e.,<br />
<br />
b<br />
and<br />
<br />
a<br />
b c<br />
b<br />
a,c<br />
<br />
2.303RT<br />
F<br />
(where )<br />
a,<br />
c<br />
for anodic processes (b a,c ):<br />
i<br />
anodic<br />
i<br />
<br />
<br />
0(<br />
T , c , c )<br />
rf<br />
<br />
rf<br />
<br />
O<br />
R<br />
<br />
<br />
<br />
ba<br />
bc<br />
10 10 i0(<br />
T , cO<br />
, cR<br />
)<br />
10 0.1<br />
<br />
<br />
<br />
10<br />
<br />
b<br />
a<br />
or, more commonly:<br />
log( i ) log<br />
0( , , )<br />
anodic<br />
1<br />
i<br />
rf<br />
<br />
T<br />
c<br />
O<br />
c<br />
R<br />
b<br />
a<br />
for cathodic processes (b a,c ): i ) logi<br />
rf<br />
<br />
<br />
log(<br />
)<br />
cathodic<br />
0( T , c<br />
O<br />
, c<br />
R<br />
1<br />
b<br />
c<br />
the log(i) vs. relationship at high is commonly referred to as Tafel equation<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 66
Butler-Volmer: Tafel Approximation<br />
for the previous example of HOR/HER on Pt(110) and Pt(111):<br />
HOR/HER on Pt(hkl) (0.05M H 2 SO 4 at 60°C)<br />
Pt face (110) (100) (111)<br />
i 0 [mA/cm 2 ] 1.35 0.76 0.83<br />
b [mV/decade] 33 44 66<br />
(Markovic et al., J. Phys. Chem. B 101 (1997) 5405))<br />
log( | |i| i | ) [ mA/cm 2 Pt ]<br />
100<br />
10<br />
1<br />
0.1<br />
(b (b a ) -1<br />
c ) -<br />
Pt(110)<br />
Pt(111)<br />
2H + + 2e - H H 2 2H + + 2e -<br />
2<br />
(i 0 rf )<br />
-100 -80 -60 -40 -20 0 20 40 60 80 100<br />
overpotential [mV]<br />
the accuracy of the Tafel equation<br />
at || ½b a,c is better 10%<br />
from Tafel plots, both (i 0 rf) and<br />
b a,c can be determined<br />
for slow kinetics, determination of<br />
(i 0<br />
rf) requires extrapolation over<br />
many orders of magnitude<br />
large errors for ORR in PEMFCs<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 67
HOR/HER Kinetic Models<br />
possible HOR reaction mech.:<br />
(K. Krischer & E.R. Savinova; in: Handbook<br />
of Het. Catalysis; Wiley (2007): ch. 8.1.1.)<br />
Tafel: H 2<br />
+ 2Pt 2 Pt-H<br />
Volmer: Pt-H Pt + H + + e -<br />
Heyrovski:<br />
H 2<br />
+ Pt Pt-H + H + + e -<br />
04k - EC SS2013 (Hubert G., Tom N., Moniek T.) p. 68