Unit 9 - Carboxylic acid Practice Problems Answers
Unit 9 - Carboxylic acid Practice Problems Answers
Unit 9 - Carboxylic acid Practice Problems Answers
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
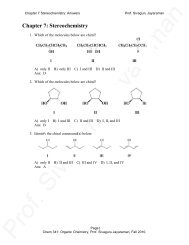
<strong>Practice</strong> 9-1<br />
Name each of the following <strong>acid</strong>s:<br />
a) CH 3 CH 2 CF 2 CH 2 COOH<br />
b) CH 3 - CH 2 - CH - COOH<br />
OH<br />
c)<br />
e)<br />
HO<br />
OH<br />
COOH<br />
NH 2<br />
CH 3 - CH - COOH<br />
d)<br />
f)<br />
COOH<br />
CH 3<br />
O<br />
OH<br />
Answer<br />
a) 3,3-difluoropentanoic <strong>acid</strong> b) 2-hydroxybutanoic <strong>acid</strong><br />
c) 3,4-dihydroxybenzoic <strong>acid</strong> d) 3-methylbenzoic <strong>acid</strong><br />
e) 2-aminopropanoic <strong>acid</strong> f) 2-ethylhexanoic <strong>acid</strong><br />
<strong>Practice</strong> 9-2<br />
Complete the following reaction and name the carboxylic <strong>acid</strong> salt formed.<br />
Answer<br />
CH 3 - CH 2 - COOH +<br />
NaOH<br />
CH 3 - CH 2 - COOH + NaOH CH 3 - CH 2 - COO - Na + + H 2 O<br />
sodium propanoate<br />
9-1
<strong>Practice</strong> 9-3<br />
Complete the following esterification reaction:<br />
O<br />
[H + ]<br />
+ CH<br />
OH<br />
3 - CH 2 -OH<br />
Answer<br />
removal of OH<br />
and H produces the ester<br />
O<br />
OH+<br />
CH 3 - CH 2 -OH<br />
[H + ]<br />
O<br />
+<br />
O- CH 2 - CH 3<br />
H 2 O<br />
<strong>Practice</strong> 9-4<br />
Write the name for each of the following esters.<br />
a)<br />
Answer<br />
c)<br />
O<br />
CH 3 (CH 2 ) 6 CH 2 COCH 3<br />
b)<br />
O<br />
OCH 2 CH 3 d)<br />
O CH 3<br />
CO CH<br />
CH 3<br />
O<br />
CH 3 CH 2 CO CH 2 CH 2 CH 3<br />
a) methyl octanate b) isopropyl benzoate<br />
c) ethyl butanoate e) propyl propanoate<br />
9-2
<strong>Practice</strong> 9-5<br />
Complete the following reactions:<br />
O<br />
CH 3 CH 2 -C-OCH 2 CH 3<br />
O<br />
CH 3 CH 2 -C-OCH 2 CH 3<br />
+ H 2 O<br />
+ NaOH<br />
H +<br />
H 2 O<br />
Answer<br />
O<br />
CH 3 CH 2 -C-OCH 2 CH 3 + H 2 O<br />
H +<br />
O<br />
CH 3 CH 2 -C-OH<br />
+ CH 3 CH 2 OH<br />
O<br />
CH 3 CH 2 -C-OCH 2 CH 3 + NaOH<br />
H 2 O<br />
O<br />
CH 3 CH 2 -C-O - Na +<br />
+ CH 3 CH 2 OH<br />
<strong>Practice</strong> 9-6<br />
Name each of the following:<br />
NH 2<br />
Br<br />
a)<br />
NH 2<br />
b)<br />
Br<br />
c)<br />
CH 3 (CH 2 ) 5 -NH-CH 2 CH 3<br />
d)<br />
N<br />
Answer<br />
a) cyclopentylamine b) 3,4-dibromoaniline<br />
c) ethylhexylamine d) diethylmethylamine<br />
9-3
<strong>Practice</strong> 9-7<br />
Name each of the following amides:<br />
O<br />
O H<br />
a) CH 3 - C - N<br />
b)<br />
C - NH - CH 2 -CH 3<br />
c) CH 3 - CH 2 - CH 3 - CH 2 - C - NH<br />
O<br />
Answer<br />
a) N-cyclohexylacetamide b) N-ethylbenzamide<br />
c) N-isopropylpentanamide<br />
<strong>Practice</strong> 9-8<br />
Draw the structures of the products in each of the following hydrolysis reactions.<br />
O<br />
C - NH 2<br />
+ H 2 O + HCl<br />
heat<br />
Answer<br />
O<br />
CH 3 - CH 2 - C - NH - CH 3<br />
+ NaOH<br />
heat<br />
O<br />
O<br />
C NH 2<br />
C<br />
OH<br />
+ H 2 O + HCl<br />
heat<br />
+ NH 4 + Cl -<br />
O<br />
CH 3 - CH 2 - C - NH - CH 3 + NaOH<br />
heat<br />
O<br />
CH 3 - CH 2 - C - O - Na +<br />
+<br />
CH 3 -NH 2<br />
9-4