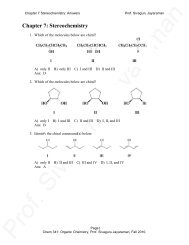
diene
diene
diene
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
DIENES<br />
Dienes are alkenes with 2 double bonds.<br />
IUPAC: Same as alkene, but change -ene to -a<strong>diene</strong> and<br />
use two numbers to locate the two double bonds (number<br />
from the end of the chain which makes the smaller of<br />
these numbers smaller).<br />
Double bonds separated by more than one single bond are<br />
isolated. Compounds with isolated double bonds have the<br />
same chemical properties as alkenes.<br />
Double bonds that alternate with single bonds,<br />
eg C=C-C=C, are conjugated.<br />
Double bond arrangements like C=C=C are cumulated.<br />
Compounds containing two carbon-carbon cumulated<br />
double bonds are called allenes.<br />
Conjugated <strong>diene</strong>s differ from simple alkenes in that they<br />
are more stable,<br />
undergo 1,4-addition, and<br />
are more reactive.
Stability<br />
Alkene Type<br />
RCH=CH 2<br />
∆H hydrogenation<br />
30 kcal/mole,exotherm.<br />
R 2 C=CH 2 , RCH=CHR 28<br />
R 2 C=CHR 27<br />
For <strong>diene</strong>s with isolated double bonds, the ∆H hydrogenation<br />
calculated by adding the appropriate values from the table<br />
above together is very close to the experimental value.<br />
For conjugated <strong>diene</strong>s, the experimental ∆H hydrogenation is<br />
smaller than the calculated one, which is based on isolated<br />
double bonds.<br />
Conjugated Diene<br />
Calculated<br />
∆H h<br />
Experimental<br />
∆H h<br />
CH 2 =CH-CH=CH 2 60 kcal/mole 57<br />
CH 2 =C(CH 3 )-C(CH 3 )=CH 2 56 54
Therefore, conjugated <strong>diene</strong>s are more stable than<br />
isolated <strong>diene</strong>s.<br />
This stability is reflected in the fact that, where possible,<br />
conjugated <strong>diene</strong>s are the preferred <strong>diene</strong> products of<br />
elimination reactions ---<br />
H<br />
H H<br />
C<br />
H<br />
C<br />
C C<br />
C<br />
Cl H<br />
KOH, alcohol<br />
heat<br />
Formation of the conjugated<br />
<strong>diene</strong> takes precedence over<br />
the Saytzeff rule.<br />
+<br />
H<br />
C<br />
C C<br />
C C<br />
H<br />
major product - conjugated<br />
H H<br />
C<br />
C<br />
C<br />
C<br />
C<br />
+ H 2 O + KCl<br />
H<br />
minor product - isolated<br />
(Saytzeff product)<br />
This stability can be explained by electron delocalization in<br />
the conjugated <strong>diene</strong><br />
using the resonance model ---<br />
or the molecular orbital model ---
Energy (Huckel) of Two Isolated vs Conjugated Double Bonds<br />
1.68<br />
_<br />
π4<br />
1.0<br />
_<br />
0.68<br />
_<br />
Energy 0<br />
-0.68<br />
_<br />
-1.0<br />
_<br />
π∗<br />
π<br />
π3<br />
π2<br />
-1.68<br />
_<br />
Two isolated π-bonds.<br />
Conjugated π-system.<br />
π1<br />
Eπ = −4.0 Eπ = −4.72
Electrophilic Addition to Conjugated Dienes:<br />
1,2- vs. 1,4- Addition ---<br />
1<br />
2<br />
3<br />
4<br />
YZ<br />
1<br />
Y<br />
2 3<br />
Z<br />
4<br />
1<br />
Y<br />
2<br />
3 4<br />
Z<br />
1,2-addition<br />
1,4-addition<br />
Note the "shift"<br />
of the π-bond.<br />
eg CH 3 CH=CH-CH=CHCH 3 + HCl ><br />
CH 3 -CH 2 -CHCl-CH=CHCH 3 via 1,2-addition<br />
+<br />
CH 3 -CH 2 CH=CH-CHCl-CH 3 via 1,4-addition
Mechanism ---<br />
H H<br />
H H<br />
4 CH<br />
1<br />
3 HCl<br />
2 4 CH<br />
CH<br />
3<br />
3<br />
CH<br />
1 3<br />
3<br />
2<br />
3<br />
H H<br />
H H H<br />
ordinary 2o carbocation -<br />
VERY LITTLE FORMED<br />
+<br />
H H<br />
H H<br />
1<br />
2 4 CH<br />
1<br />
3 3<br />
2<br />
CH 3 4<br />
CH 3<br />
3<br />
CH 3<br />
H H<br />
H H<br />
H H<br />
2o allylic type of carbocation; ~ stability of 3o carbocation.<br />
Since the positive charge is shared between the carbons labeled 2 & 4<br />
Cl - can attack either.
1,2- vs. 1,4-Addition: Rate vs. Equilibrium Control<br />
1<br />
2<br />
3<br />
4<br />
HBr<br />
Low temp (-80o C)<br />
High temp (40o C)<br />
1<br />
2 3<br />
H Br<br />
1<br />
2<br />
3 4<br />
1<br />
4<br />
2<br />
80%<br />
40oC<br />
3 4<br />
1<br />
2 4<br />
3<br />
80%<br />
H 20% H 20%<br />
Br<br />
Br<br />
H<br />
Br<br />
At -80o the 1,2-isomer<br />
converts to 1,4-isomer<br />
very slowly. Since 1,2-<br />
predominates here,<br />
it is formed faster.<br />
Reaction rates determine<br />
product here:<br />
Kinetic Control<br />
At 40o the 1,2- and 1,4-<br />
isomers interconvert, giving<br />
an equilibrium mixture.<br />
The 1,4-isomer is more<br />
stable and predominates.<br />
Equilibrium constant<br />
determines product here:<br />
Thermodynamic Control<br />
In this case, the less stable product is formed faster.<br />
How does this happen
Diels-Alder Reaction<br />
X<br />
Y<br />
X<br />
Y<br />
<strong>diene</strong><br />
<strong>diene</strong>ophile<br />
(X, Y: typically<br />
one or both are<br />
electron<br />
withdrawing)<br />
a substituted cyclohexene<br />
There are other variations on this theme, eg---<br />
X<br />
X<br />
Y<br />
Y
Stereochemistry ---<br />
1) With respect to the <strong>diene</strong>ophile, the addition is<br />
stereospecifically syn, eg<br />
H<br />
Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
H<br />
Cl<br />
Cl<br />
H<br />
H<br />
Cl<br />
Cl<br />
H<br />
+<br />
Cl<br />
H<br />
H<br />
Cl<br />
2) With respect to the <strong>diene</strong>, the addition is<br />
stereospecific and syn, eg<br />
C 6 H 5<br />
C 6 H 5<br />
Cl Cl<br />
Cl Cl C 6 H 5<br />
Cl Cl<br />
Cl Cl<br />
C 6 H 5
3) The <strong>diene</strong> must be in the s-cis conformation for<br />
reaction to occur. [The mechanism is concerted --- no<br />
intermediates.]<br />
close<br />
enough<br />
s-cis<br />
close<br />
enough<br />
too<br />
far<br />
s-trans<br />
too<br />
far<br />
Dienes unable to attain the s-cis conformation do not react, eg
4) If the <strong>diene</strong> is cyclic and the <strong>diene</strong>ophile has an<br />
unsaturated substituent, the endo product is usually<br />
favored over the exo product [the reaction is<br />
stereoselective; one diasteromer (endo) is favored<br />
over another (exo)].<br />
a<br />
3<br />
4<br />
cyclopenta<strong>diene</strong><br />
3<br />
4<br />
b<br />
e<br />
2 2<br />
5<br />
2<br />
5<br />
d<br />
c<br />
c<br />
d<br />
1<br />
1<br />
b<br />
cyclopenta<strong>diene</strong><br />
e<br />
a<br />
4<br />
3<br />
1<br />
5<br />
d<br />
H<br />
H<br />
exo-dicyclopenta<strong>diene</strong><br />
4<br />
3<br />
1<br />
5<br />
2<br />
H<br />
d<br />
c<br />
H<br />
e<br />
endo-dicyclopenta<strong>diene</strong><br />
e<br />
c<br />
b<br />
b<br />
a<br />
a