Gap Analysis
Gap Analysis
Gap Analysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
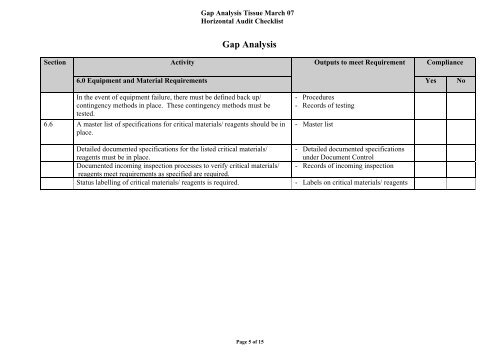
<strong>Gap</strong> <strong>Analysis</strong> Tissue March 07<br />
Horizontal Audit Checklist<br />
<strong>Gap</strong> <strong>Analysis</strong><br />
Section<br />
Activity<br />
Outputs to meet Requirement<br />
Compliance<br />
6.0 Equipment and Material Requirements<br />
Yes<br />
No<br />
In the event of equipment failure, there must be defined back up/<br />
contingency methods in place. These contingency methods must be<br />
tested.<br />
6.6 A master list of specifications for critical materials/ reagents should be in<br />
place.<br />
- Procedures<br />
- Records of testing<br />
- Master list<br />
Detailed documented specifications for the listed critical materials/ - Detailed documented specifications<br />
reagents must be in place.<br />
under Document Control<br />
Documented incoming inspection processes to verify critical materials/ - Records of incoming inspection<br />
reagents meet requirements as specified are required.<br />
Status labelling of critical materials/ reagents is required. - Labels on critical materials/ reagents<br />
Page 5 of 15