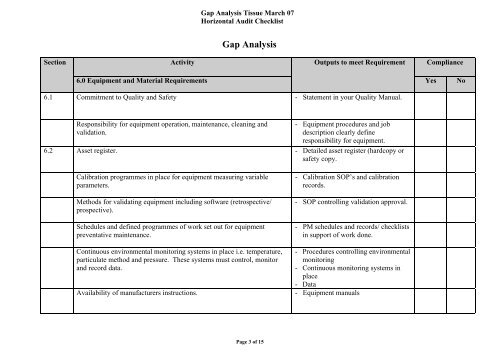
Gap Analysis
Gap Analysis
Gap Analysis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Gap</strong> <strong>Analysis</strong> Tissue March 07<br />
Horizontal Audit Checklist<br />
<strong>Gap</strong> <strong>Analysis</strong><br />
Section<br />
Activity<br />
Outputs to meet Requirement<br />
Compliance<br />
6.0 Equipment and Material Requirements<br />
6.1 Commitment to Quality and Safety - Statement in your Quality Manual.<br />
Yes<br />
No<br />
Responsibility for equipment operation, maintenance, cleaning and<br />
validation.<br />
- Equipment procedures and job<br />
description clearly define<br />
responsibility for equipment.<br />
6.2 Asset register. - Detailed asset register (hardcopy or<br />
safety copy.<br />
Calibration programmes in place for equipment measuring variable<br />
parameters.<br />
Methods for validating equipment including software (retrospective/<br />
prospective).<br />
Schedules and defined programmes of work set out for equipment<br />
preventative maintenance.<br />
- Calibration SOP’s and calibration<br />
records.<br />
- SOP controlling validation approval.<br />
- PM schedules and records/ checklists<br />
in support of work done.<br />
Continuous environmental monitoring systems in place i.e. temperature,<br />
particulate method and pressure. These systems must control, monitor<br />
and record data.<br />
- Procedures controlling environmental<br />
monitoring<br />
- Continuous monitoring systems in<br />
place<br />
- Data<br />
Availability of manufacturers instructions. - Equipment manuals<br />
Page 3 of 15