A Review of Recent Progress in the Design and ... - Neue Verpackung
A Review of Recent Progress in the Design and ... - Neue Verpackung
A Review of Recent Progress in the Design and ... - Neue Verpackung
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
production are low polymerization rates<br />
without microemulsification <strong>and</strong> an <strong>in</strong>sufficient<br />
reduction <strong>in</strong> <strong>the</strong> number <strong>of</strong> selectively<br />
acidic VF2 sites for high resistance toward<br />
bases. This occurs because ethylene<br />
does not readily add to a propagat<strong>in</strong>g VF2<br />
radical but <strong>in</strong>stead prefers to add to a propagat<strong>in</strong>g<br />
TFE radical, <strong>and</strong> <strong>the</strong> preference<br />
for VF2 by propagat<strong>in</strong>g HFP radicals rema<strong>in</strong>s<br />
high. Practical base resistance tests<br />
<strong>in</strong> hot, aggressively formulated motor oils<br />
show only moderate improvements compared<br />
to st<strong>and</strong>ard FKM products by this<br />
ethylene dilution strategy.<br />
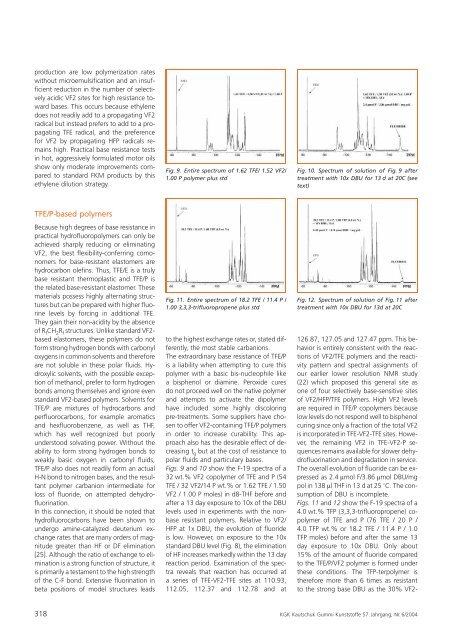
Fig. 9. Entire spectrum <strong>of</strong> 1.62 TFE/ 1.52 VF2/<br />
1.00 P polymer plus std<br />
Fig. 10. Spectrum <strong>of</strong> solution <strong>of</strong> Fig. 9 after<br />
treatment with 10x DBU for 13 d at 20C (see<br />
text)<br />
TFE/P-based polymers<br />
Because high degrees <strong>of</strong> base resistance <strong>in</strong><br />
practical hydr<strong>of</strong>luoropolymers can only be<br />
achieved sharply reduc<strong>in</strong>g or elim<strong>in</strong>at<strong>in</strong>g<br />
VF2, <strong>the</strong> best flexibility-conferr<strong>in</strong>g comonomers<br />
for base-resistant elastomers are<br />
hydrocarbon olef<strong>in</strong>s. Thus, TFE/E is a truly<br />
base resistant <strong>the</strong>rmoplastic <strong>and</strong> TFE/P is<br />
<strong>the</strong> related base-resistant elastomer. These<br />
materials possess highly alternat<strong>in</strong>g structures<br />
but can be prepared with higher fluor<strong>in</strong>e<br />
levels by forc<strong>in</strong>g <strong>in</strong> additional TFE.<br />
They ga<strong>in</strong> <strong>the</strong>ir non-acidity by <strong>the</strong> absence<br />
<strong>of</strong> R f CH 2 R f structures. Unlike st<strong>and</strong>ard VF2-<br />
based elastomers, <strong>the</strong>se polymers do not<br />
form strong hydrogen bonds with carbonyl<br />
oxygens <strong>in</strong> common solvents <strong>and</strong> <strong>the</strong>refore<br />
are not soluble <strong>in</strong> <strong>the</strong>se polar fluids. Hydroxylic<br />
solvents, with <strong>the</strong> possible exception<br />
<strong>of</strong> methanol, prefer to form hydrogen<br />
bonds among <strong>the</strong>mselves <strong>and</strong> ignore even<br />
st<strong>and</strong>ard VF2-based polymers. Solvents for<br />
TFE/P are mixtures <strong>of</strong> hydrocarbons <strong>and</strong><br />
perfluorocarbons, for example aromatics<br />
<strong>and</strong> hexfluorobenzene, as well as THF,<br />
which has well recognized but poorly<br />
understood solvat<strong>in</strong>g power. Without <strong>the</strong><br />
ability to form strong hydrogen bonds to<br />
weakly basic oxygen <strong>in</strong> carbonyl fluids,<br />
TFE/P also does not readily form an actual<br />
H-N bond to nitrogen bases, <strong>and</strong> <strong>the</strong> resultant<br />
polymer carbanion <strong>in</strong>termediate for<br />
loss <strong>of</strong> fluoride, on attempted dehydr<strong>of</strong>luor<strong>in</strong>ation.<br />
In this connection, it should be noted that<br />
hydr<strong>of</strong>luorocarbons have been shown to<br />
undergo am<strong>in</strong>e-catalyzed deuterium exchange<br />
rates that are many orders <strong>of</strong> magnitude<br />
greater than HF or DF elim<strong>in</strong>ation<br />
[25]. Although <strong>the</strong> ratio <strong>of</strong> exchange to elim<strong>in</strong>ation<br />
is a strong function <strong>of</strong> structure, it<br />
is primarily a testament to <strong>the</strong> high strength<br />
<strong>of</strong> <strong>the</strong> C-F bond. Extensive fluor<strong>in</strong>ation <strong>in</strong><br />
beta positions <strong>of</strong> model structures leads<br />
Fig. 11. Entire spectrum <strong>of</strong> 18.2 TFE / 11.4 P /<br />
1.00 3,3,3-trifluoropropene plus std<br />
to <strong>the</strong> highest exchange rates or, stated differently,<br />
<strong>the</strong> most stable carbanions.<br />
The extraord<strong>in</strong>ary base resistance <strong>of</strong> TFE/P<br />
is a liability when attempt<strong>in</strong>g to cure this<br />
polymer with a basic bis-nucleophile like<br />
a bisphenol or diam<strong>in</strong>e. Peroxide cures<br />
do not proceed well on <strong>the</strong> native polymer<br />
<strong>and</strong> attempts to activate <strong>the</strong> dipolymer<br />
have <strong>in</strong>cluded some highly discolor<strong>in</strong>g<br />
pre-treatments. Some suppliers have chosen<br />
to <strong>of</strong>fer VF2-conta<strong>in</strong><strong>in</strong>g TFE/P polymers<br />
<strong>in</strong> order to <strong>in</strong>crease curability. This approach<br />
also has <strong>the</strong> desirable effect <strong>of</strong> decreas<strong>in</strong>g<br />
t g but at <strong>the</strong> cost <strong>of</strong> resistance to<br />
polar fluids <strong>and</strong> particulary bases.<br />
Figs. 9 <strong>and</strong> 10 show <strong>the</strong> F-19 spectra <strong>of</strong> a<br />
32 wt.% VF2 copolymer <strong>of</strong> TFE <strong>and</strong> P (54<br />
TFE / 32 VF2/14 P wt.% or 1.62 TFE / 1.50<br />
VF2 / 1.00 P moles) <strong>in</strong> d8-THF before <strong>and</strong><br />
after a 13 day exposure to 10x <strong>of</strong> <strong>the</strong> DBU<br />
levels used <strong>in</strong> experiments with <strong>the</strong> nonbase<br />
resistant polymers. Relative to VF2/<br />
HFP at 1x DBU, <strong>the</strong> evolution <strong>of</strong> fluoride<br />
is low. However, on exposure to <strong>the</strong> 10x<br />
st<strong>and</strong>ard DBU level (Fig. 8), <strong>the</strong> elim<strong>in</strong>ation<br />
<strong>of</strong> HF <strong>in</strong>creases markedly with<strong>in</strong> <strong>the</strong> 13 day<br />
reaction period. Exam<strong>in</strong>ation <strong>of</strong> <strong>the</strong> spectra<br />
reveals that reaction has occurred at<br />
a series <strong>of</strong> TFE-VF2-TFE sites at 110.93,<br />
112.05, 112.37 <strong>and</strong> 112.78 <strong>and</strong> at<br />
Fig. 12. Spectrum <strong>of</strong> solution <strong>of</strong> Fig. 11 after<br />
treatment with 10x DBU for 13d at 20C<br />
126.87, 127.05 <strong>and</strong> 127.47 ppm. This behavior<br />
is entirely consistent with <strong>the</strong> reactions<br />
<strong>of</strong> VF2/TFE polymers <strong>and</strong> <strong>the</strong> reactivity<br />
pattern <strong>and</strong> spectral assignments <strong>of</strong><br />
our earlier lower resolution NMR study<br />
(22) which proposed this general site as<br />
one <strong>of</strong> four selectively base-sensitive sites<br />
<strong>of</strong> VF2/HFP/TFE polymers. High VF2 levels<br />
are required <strong>in</strong> TFE/P copolymers because<br />
low levels do not respond well to bisphenol<br />
cur<strong>in</strong>g s<strong>in</strong>ce only a fraction <strong>of</strong> <strong>the</strong> total VF2<br />
is <strong>in</strong>corporated <strong>in</strong> TFE-VF2-TFE sites. However,<br />
<strong>the</strong> rema<strong>in</strong><strong>in</strong>g VF2 <strong>in</strong> TFE-VF2-P sequences<br />
rema<strong>in</strong>s available for slower dehydr<strong>of</strong>luor<strong>in</strong>ation<br />
<strong>and</strong> degradation <strong>in</strong> service.<br />
The overall evolution <strong>of</strong> fluoride can be expressed<br />
as 2.4 lmol F/3.86 lmol DBU/mg<br />
pol <strong>in</strong> 138 ll THF <strong>in</strong> 13 d at 25 8C. The consumption<br />
<strong>of</strong> DBU is <strong>in</strong>complete.<br />
Figs. 11 <strong>and</strong> 12 show <strong>the</strong> F-19 spectra <strong>of</strong> a<br />
4.0 wt.% TFP (3,3,3-trifluoropropene) copolymer<br />
<strong>of</strong> TFE <strong>and</strong> P (76 TFE / 20 P /<br />
4.0 TFP wt.% or 18.2 TFE / 11.4 P / 1.0<br />
TFP moles) before <strong>and</strong> after <strong>the</strong> same 13<br />
day exposure to 10x DBU. Only about<br />
15% <strong>of</strong> <strong>the</strong> amount <strong>of</strong> fluoride compared<br />
to <strong>the</strong> TFE/P/VF2 polymer is formed under<br />
<strong>the</strong>se conditions. The TFP-terpolymer is<br />
<strong>the</strong>refore more than 6 times as resistant<br />
to <strong>the</strong> strong base DBU as <strong>the</strong> 30% VF2-<br />
318 KGK Kautschuk Gummi Kunstst<strong>of</strong>fe 57. Jahrgang, Nr. 6/2004