Unit 1 Module 3 The Periodic Table - Pearson Schools
Unit 1 Module 3 The Periodic Table - Pearson Schools
Unit 1 Module 3 The Periodic Table - Pearson Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
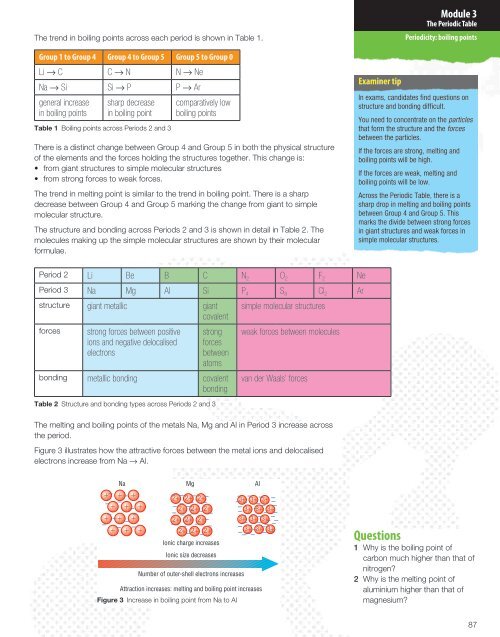
<strong>The</strong> trend in boiling points across each period is shown in <strong>Table</strong> 1.<br />
Group 1 to Group 4 Group 4 to Group 5 Group 5 to Group 0<br />
Li → C C → N N → Ne<br />
Na → Si Si → P P → Ar<br />
general increase<br />
in boiling points<br />
sharp decrease<br />
in boiling point<br />
<strong>Table</strong> 1 Boiling points across Periods 2 and 3<br />
comparatively low<br />
boiling points<br />
<strong>The</strong>re is a distinct change between Group 4 and Group 5 in both the physical structure<br />
of the elements and the forces holding the structures together. This change is:<br />
• from giant structures to simple molecular structures<br />
• from strong forces to weak forces.<br />
<strong>The</strong> trend in melting point is similar to the trend in boiling point. <strong>The</strong>re is a sharp<br />
decrease between Group 4 and Group 5 marking the change from giant to simple<br />
molecular structure.<br />
<strong>The</strong> structure and bonding across Periods 2 and 3 is shown in detail in <strong>Table</strong> 2. <strong>The</strong><br />
molecules making up the simple molecular structures are shown by their molecular<br />
formulae.<br />
Examiner tip<br />
<strong>Module</strong> 3<br />
<strong>The</strong> <strong>Periodic</strong> <strong>Table</strong><br />
<strong>Periodic</strong>ity: boiling points<br />
In ex, fi <br />
fi.<br />
Y particles<br />
forces<br />
p.<br />
I , <br />
boiling p .<br />
I k, <br />
boiling p .<br />
A <strong>Table</strong>, <br />
p drop p<br />
Group 4 and Group 5. T<br />
mark <br />
k <br />
p .<br />
Period 2 Li Be B C N 2 O 2 F 2 Ne<br />
Period 3 Na Mg Al Si P 4 S 8 Cl 2 Ar<br />
structure giant metallic giant<br />
covalent<br />
forces<br />
strong forces between positive<br />
ions and negative delocalised<br />
electrons<br />
strong<br />
forces<br />
between<br />
atoms<br />
bonding metallic bonding covalent<br />
bonding<br />
<strong>Table</strong> 2 Structure and bonding types across Periods 2 and 3<br />
simple molecular structures<br />
weak forces between molecules<br />
van der Waals’ forces<br />
<strong>The</strong> melting and boiling points of the metals Na, Mg and Al in Period 3 increase across<br />
the period.<br />
Figure 3 illustrates how the attractive forces between the metal ions and delocalised<br />
electrons increase from Na → Al.<br />
Na Mg Al<br />
+ − + − +<br />
2+<br />
− − 2+ − 2+ −<br />
− −<br />
−<br />
+ + +<br />
− −<br />
− − −3+ 3+ 3+<br />
−<br />
− 2+ −<br />
− − − − −−<br />
2+ 2+<br />
+<br />
− − + +<br />
− − −<br />
−<br />
−<br />
− 3+ 3+ −<br />
− − −<br />
− 3+<br />
− −<br />
−<br />
2+ 2+ − 2+ − 3+ 3+ − − 3+ −<br />
−<br />
+<br />
−<br />
+<br />
− − − − − − −<br />
− − − −<br />
+<br />
2+ − 2+ 2+<br />
3+ 3+ 3+<br />
− −− −− − − − −<br />
Ionic charge increases<br />
Ionic size decreases<br />
Number of outer-shell electrons increases<br />
Attraction increases: melting and boiling point increases<br />
Figure 3 Increase in boiling point from Na to Al<br />
Questions<br />
1 Why is the boiling point of<br />
carbon much higher than that of<br />
nitrogen?<br />
2 Why is the melting point of<br />
aluminium higher than that of<br />
magnesium?<br />
87<br />
935 chemistry.U1 M3.indd 87 13/11/07 11:50:05 am