Unit 1 Module 3 The Periodic Table - Pearson Schools
Unit 1 Module 3 The Periodic Table - Pearson Schools
Unit 1 Module 3 The Periodic Table - Pearson Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
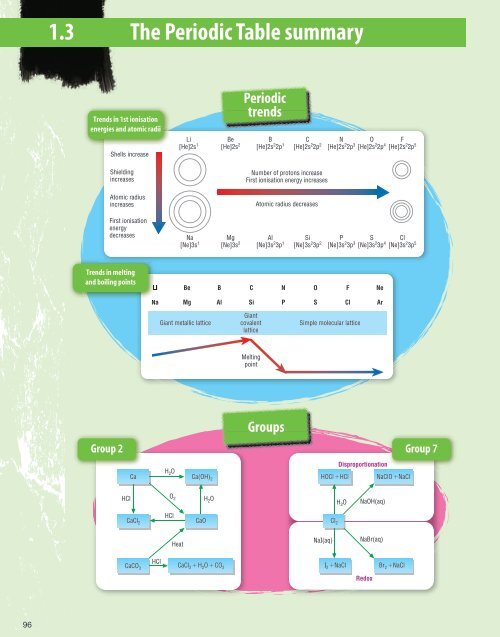
1.3 <strong>The</strong> <strong>Periodic</strong> <strong>Table</strong> summary<br />
Trends in 1st ionisation<br />
energies and atomic radii<br />
Shells increase<br />
Li<br />
[He]2s 1<br />
Be<br />
[He]2s 2<br />
<strong>Periodic</strong><br />
trends<br />
B<br />
[He]2s 2 2p 1<br />
C<br />
[He]2s 2 2p 2<br />
N<br />
[He]2s 2 2p 3<br />
O<br />
[He]2s 2 2p 4<br />
F<br />
[He]2s 2 2p 5<br />
Shielding<br />
increases<br />
Atomic radius<br />
increases<br />
Number of protons increase<br />
First ionisation energy increases<br />
Atomic radius decreases<br />
First ionisation<br />
energy<br />
decreases<br />
Na<br />
[Ne]3s 1<br />
Mg<br />
[Ne]3s 2<br />
Al<br />
[Ne]3s 2 3p 1<br />
Si<br />
[Ne]3s 2 3p 2<br />
P<br />
[Ne]3s 2 3p 3<br />
S<br />
[Ne]3s 2 3p 4<br />
Cl<br />
[Ne]3s 2 3p 5<br />
Trends in melting<br />
and boiling points<br />
Li<br />
Be<br />
B<br />
C<br />
N<br />
O<br />
F<br />
Ne<br />
Na<br />
Mg<br />
Al<br />
Si<br />
P<br />
S<br />
Cl<br />
Ar<br />
Giant metallic lattice<br />
Giant<br />
covalent<br />
lattice<br />
Simple molecular lattice<br />
Melting<br />
point<br />
Groups<br />
Group 2<br />
Group 7<br />
Ca<br />
H 2 O<br />
Ca(OH) 2<br />
Disproportionation<br />
HOCl HCl NaClO NaCl<br />
HCl O 2<br />
H 2 O<br />
H 2 O<br />
NaOH(aq)<br />
CaCl 2<br />
CaCl 2 H 2 O CO 2<br />
HCl<br />
CaO<br />
Cl 2<br />
Heat<br />
NaI(aq)<br />
NaBr(aq)<br />
CaCO 3<br />
HCl<br />
I 2 NaCl<br />
Br 2 NaCl<br />
Redox<br />
96<br />
935 chemistry.U1 M3.indd 96 13/11/07 11:51:20 am