PHYSICS 455 Assignment 3 Due February 15, 2000 1. A simple ...
PHYSICS 455 Assignment 3 Due February 15, 2000 1. A simple ...
PHYSICS 455 Assignment 3 Due February 15, 2000 1. A simple ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>PHYSICS</strong> <strong>455</strong><br />
<strong>Assignment</strong> 3<br />
<strong>Due</strong> <strong>February</strong> <strong>15</strong>, <strong>2000</strong><br />
<strong>1.</strong> A <strong>simple</strong> model of rubber elasticity. Consider a polymer chain of N rigid links, but with<br />
flexible joints between the junctions. Each link has a length a, and can point independently<br />
along any of the x, −x, y, −y, z, −z axes (yes, it can point back on itself). The entire assembly<br />
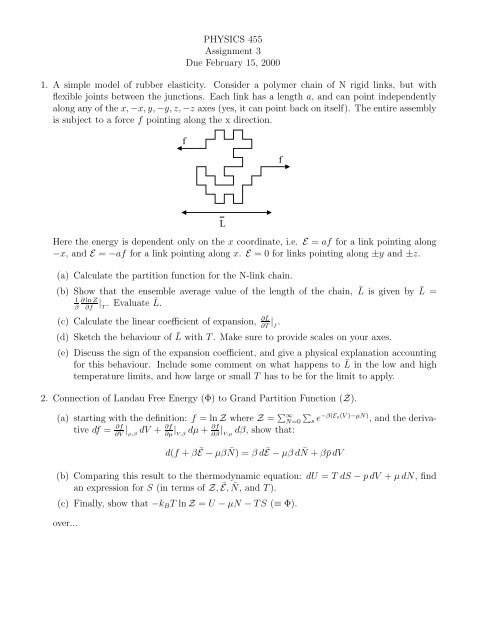
is subject to a force f pointing along the x direction.<br />
f<br />
f<br />
L<br />
Here the energy is dependent only on the x coordinate, i.e. E = af for a link pointing along<br />
−x, and E = −af for a link pointing along x. E = 0 for links pointing along ±y and ±z.<br />
(a) Calculate the partition function for the N-link chain.<br />
(b) Show that the ensemble average value of the length of the chain, ¯L is given by ¯L =<br />
. Evaluate ¯L.<br />
1<br />
β<br />
∂ ln Z<br />
∂f<br />
| T<br />
(c) Calculate the linear coefficient of expansion, ∂ ¯L<br />
∂T | f .<br />
(d) Sketch the behaviour of ¯L with T . Make sure to provide scales on your axes.<br />
(e) Discuss the sign of the expansion coefficient, and give a physical explanation accounting<br />
for this behaviour. Include some comment on what happens to ¯L in the low and high<br />
temperature limits, and how large or small T has to be for the limit to apply.<br />
2. Connection of Landau Free Energy (Φ) to Grand Partition Function (Z).<br />
(a) starting with the definition: f = ln Z where Z = ∑ ∞<br />
N=0<br />
∑s e −β(Es(V )−µN) , and the derivative<br />
df = ∂f | ∂f<br />
dV + | ∂f<br />
dµ + | dβ, show that:<br />
∂V µ,β ∂µ V,β ∂β V,µ<br />
d(f + βĒ − µβ ¯N) = β dĒ − µβ d ¯N + β ¯p dV<br />
(b) Comparing this result to the thermodynamic equation: dU = T dS − p dV + µ dN, find<br />
an expression for S (in terms of Z, Ē, ¯N, and T ).<br />
(c) Finally, show that −k B T ln Z = U − µN − T S (≡ Φ).<br />
over...
3. Consider a system consisting of a single impurity atom/ion in a semiconductor. Suppose that<br />
the impurity atom has one “extra” electron compared to the neighboring atoms, as would a<br />
phosphorus atom occupying a lattice site in a silicon crystal. The extra electron is then easily<br />
removed, leaving behind a positively charged ion. The ionized electron is called a conduction<br />
electron, because it is free to move through the material; the impurity atom is called a donor,<br />
because it can “donate” a conduction electron.<br />
(a) Derive a formula for the probability of a single donor atom being ionized. Do not neglect<br />
the fact that the electron, if present, can have two independent spin states. Express your<br />
formula in terms of the temperature, the ionization energy I, and the chemical potential<br />
of the “gas” of conduction electrons.<br />
(b) Assuming that the conduction electrons behave like an ordinary ideal gas (with two spin<br />
states per particle), write their chemical potential in terms of the number of conduction<br />
electrons per unit volume, N c /V .<br />
(c) Now assume that every conduction electron comes from an ionized donor atom. In<br />
this case the number of conduction electrons is equal to the number of donors that are<br />
ionized. Use this condition to derive a quadratic equation for N c in terms of the number<br />
of donor atoms (N d ), eliminating µ. Solve for N c using the quadratic formula. (It may<br />
be helpful to introduce some abbreviations for dimensionless quantities. Try x = N c /N d ,<br />
t = k B T/I, and so on.)<br />
4. Hemoglobin is found as assemblies of four protein subunits, with each subunit having an<br />
oxygen binding heme site. The tendency of oxygen to bind to a heme site increases as the<br />
other sites become occupied. This system may have zero, one, two, three, or four oxygen<br />
molecules bound. Taking the energy of the unbound molecule to be 0, assume that the energy<br />
of the singly occupied states is -0.55 eV, the doubly occupied states as -<strong>1.</strong>3 eV, the triply<br />
occupied states as -2.0 eV, and the quadruply occupied states as -3 eV, calculate and plot the<br />
fraction of occupied sites as a function of the effective partial pressure of oxygen. Compare<br />
to the graph we sketched in class for the independent site (Myoglobin) case.