Water in Glassy Carbohydrates: Opening It Up at the ... - Physics
Water in Glassy Carbohydrates: Opening It Up at the ... - Physics
Water in Glassy Carbohydrates: Opening It Up at the ... - Physics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>W<strong>at</strong>er</strong> <strong>in</strong> <strong>Glassy</strong> <strong>Carbohydr<strong>at</strong>es</strong> J. Phys. Chem. B, Vol. 108, No. 33, 2004 12439<br />
TABLE 2: L<strong>in</strong>ear Regression of PALS D<strong>at</strong>a below and<br />
above <strong>the</strong> Glass Transition Temper<strong>at</strong>ure a<br />
a w <strong>at</strong><br />
25 °C<br />
slope T < T g<br />
T g<br />
[Å 3 ‚K -1 ] b R 2 [°C] c<br />
slope T > T g<br />
[Å 3 ‚K -1 ] b R 2<br />
0.11 0.215 0.98 97.6 0.384 0.97<br />
0.22 0.265 0.99 86.4 0.376 0.97<br />
0.33 0.325 0.98 73.0 0.548 0.98<br />
0.54 0.362 0.89 53.7 0.503 0.97<br />
0.75 0.278 0.99 18.9 0.539 0.99<br />
a<br />
The glass transition temper<strong>at</strong>ure is determ<strong>in</strong>ed self-consistently from<br />
a l<strong>in</strong>ear regression of <strong>the</strong> d<strong>at</strong>a <strong>in</strong> <strong>the</strong> glassy and rubbery st<strong>at</strong>es. b Slope<br />
of <strong>the</strong> l<strong>in</strong>ear regression of <strong>the</strong> hole volume versus temper<strong>at</strong>ure. c Def<strong>in</strong>ed<br />
as <strong>the</strong> temper<strong>at</strong>ure of <strong>in</strong>tersection of <strong>the</strong> l<strong>in</strong>ear regression of <strong>the</strong> d<strong>at</strong>a<br />
<strong>in</strong> <strong>the</strong> rubbery and glassy st<strong>at</strong>es.<br />
Figure 3. Mean hole volume from <strong>the</strong> PALS experiments as a function<br />
of <strong>the</strong> w<strong>at</strong>er content. Correl<strong>at</strong>ion coefficient of <strong>the</strong> l<strong>in</strong>ear fit R 2 ) 0.97.<br />
T ) 25 °C.<br />
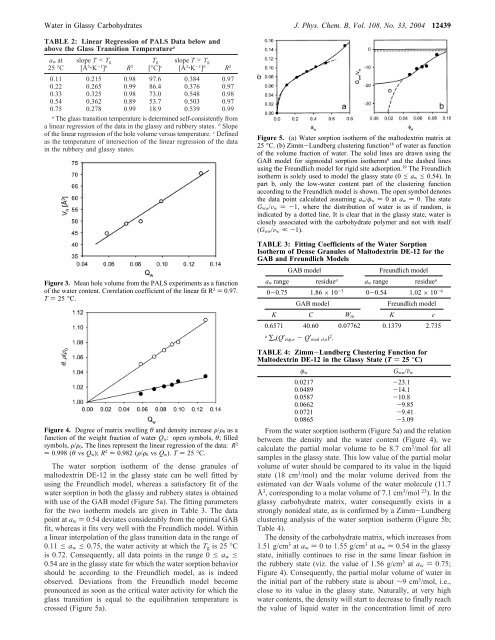
Figure 5. (a) <strong>W<strong>at</strong>er</strong> sorption iso<strong>the</strong>rm of <strong>the</strong> maltodextr<strong>in</strong> m<strong>at</strong>rix <strong>at</strong><br />
25 °C. (b) Zimm-Lundberg cluster<strong>in</strong>g function 18 of w<strong>at</strong>er as function<br />
of <strong>the</strong> volume fraction of w<strong>at</strong>er. The solid l<strong>in</strong>es are drawn us<strong>in</strong>g <strong>the</strong><br />
GAB model for sigmoidal sorption iso<strong>the</strong>rms 6 and <strong>the</strong> dashed l<strong>in</strong>es<br />
us<strong>in</strong>g <strong>the</strong> Freundlich model for rigid site adsorption. 19 The Freundlich<br />
iso<strong>the</strong>rm is solely used to model <strong>the</strong> glassy st<strong>at</strong>e (0 e a w e 0.54). In<br />
part b, only <strong>the</strong> low-w<strong>at</strong>er content part of <strong>the</strong> cluster<strong>in</strong>g function<br />
accord<strong>in</strong>g to <strong>the</strong> Freundlich model is shown. The open symbol denotes<br />
<strong>the</strong> d<strong>at</strong>a po<strong>in</strong>t calcul<strong>at</strong>ed assum<strong>in</strong>g a w/φ w ) 0<strong>at</strong>a w ) 0. The st<strong>at</strong>e<br />
G ww/V w )-1, where <strong>the</strong> distribution of w<strong>at</strong>er is as if random, is<br />
<strong>in</strong>dic<strong>at</strong>ed by a dotted l<strong>in</strong>e. <strong>It</strong> is clear th<strong>at</strong> <strong>in</strong> <strong>the</strong> glassy st<strong>at</strong>e, w<strong>at</strong>er is<br />
closely associ<strong>at</strong>ed with <strong>the</strong> carbohydr<strong>at</strong>e polymer and not with itself<br />
(G ww/V w ,-1).<br />
TABLE 3: Fitt<strong>in</strong>g Coefficients of <strong>the</strong> <strong>W<strong>at</strong>er</strong> Sorption<br />
Iso<strong>the</strong>rm of Dense Granules of Maltodextr<strong>in</strong> DE-12 for <strong>the</strong><br />
GAB and Freundlich Models<br />
GAB model<br />
Freundlich model<br />
a w range residue a a w range residue a<br />
0-0.75 1.86 × 10 -5 0-0.54 1.02 × 10 -6<br />
GAB model<br />
Freundlich model<br />
K C W m K c<br />
0.6571 40.60 0.07762 0.1379 2.735<br />
a<br />
∑ n(Q′ exp,n - Q′ mod el,n) 2 .<br />
TABLE 4: Zimm-Lundberg Cluster<strong>in</strong>g Function for<br />
Maltodextr<strong>in</strong> DE-12 <strong>in</strong> <strong>the</strong> <strong>Glassy</strong> St<strong>at</strong>e (T ) 25 °C)<br />
Figure 4. Degree of m<strong>at</strong>rix swell<strong>in</strong>g θ and density <strong>in</strong>crease F/F 0 as a<br />
function of <strong>the</strong> weight fraction of w<strong>at</strong>er Q w: open symbols, θ; filled<br />
symbols, F/F 0. The l<strong>in</strong>es represent <strong>the</strong> l<strong>in</strong>ear regression of <strong>the</strong> d<strong>at</strong>a: R 2<br />
) 0.998 (θ vs Q w); R 2 ) 0.982 (F/F 0 vs Q w). T ) 25 °C.<br />
The w<strong>at</strong>er sorption iso<strong>the</strong>rm of <strong>the</strong> dense granules of<br />
maltodextr<strong>in</strong> DE-12 <strong>in</strong> <strong>the</strong> glassy st<strong>at</strong>e can be well fitted by<br />
us<strong>in</strong>g <strong>the</strong> Freundlich model, whereas a s<strong>at</strong>isfactory fit of <strong>the</strong><br />
w<strong>at</strong>er sorption <strong>in</strong> both <strong>the</strong> glassy and rubbery st<strong>at</strong>es is obta<strong>in</strong>ed<br />
with use of <strong>the</strong> GAB model (Figure 5a). The fitt<strong>in</strong>g parameters<br />
for <strong>the</strong> two iso<strong>the</strong>rm models are given <strong>in</strong> Table 3. The d<strong>at</strong>a<br />
po<strong>in</strong>t <strong>at</strong> a w ) 0.54 devi<strong>at</strong>es considerably from <strong>the</strong> optimal GAB<br />
fit, whereas it fits very well with <strong>the</strong> Freundlich model. With<strong>in</strong><br />
a l<strong>in</strong>ear <strong>in</strong>terpol<strong>at</strong>ion of <strong>the</strong> glass transition d<strong>at</strong>a <strong>in</strong> <strong>the</strong> range of<br />
0.11 e a w e 0.75, <strong>the</strong> w<strong>at</strong>er activity <strong>at</strong> which <strong>the</strong> T g is 25 °C<br />
is 0.72. Consequently, all d<strong>at</strong>a po<strong>in</strong>ts <strong>in</strong> <strong>the</strong> range 0 e a w e<br />
0.54 are <strong>in</strong> <strong>the</strong> glassy st<strong>at</strong>e for which <strong>the</strong> w<strong>at</strong>er sorption behavior<br />
should be accord<strong>in</strong>g to <strong>the</strong> Freundlich model, as is <strong>in</strong>deed<br />
observed. Devi<strong>at</strong>ions from <strong>the</strong> Freundlich model become<br />
pronounced as soon as <strong>the</strong> critical w<strong>at</strong>er activity for which <strong>the</strong><br />
glass transition is equal to <strong>the</strong> equilibr<strong>at</strong>ion temper<strong>at</strong>ure is<br />
crossed (Figure 5a).<br />
φ w<br />
G ww/Vj w<br />
0.0217 -23.1<br />
0.0489 -14.1<br />
0.0587 -10.8<br />
0.0662 -9.85<br />
0.0721 -9.41<br />
0.0865 -3.09<br />
From <strong>the</strong> w<strong>at</strong>er sorption iso<strong>the</strong>rm (Figure 5a) and <strong>the</strong> rel<strong>at</strong>ion<br />
between <strong>the</strong> density and <strong>the</strong> w<strong>at</strong>er content (Figure 4), we<br />
calcul<strong>at</strong>e <strong>the</strong> partial molar volume to be 8.7 cm 3 /mol for all<br />
samples <strong>in</strong> <strong>the</strong> glassy st<strong>at</strong>e. This low value of <strong>the</strong> partial molar<br />
volume of w<strong>at</strong>er should be compared to its value <strong>in</strong> <strong>the</strong> liquid<br />
st<strong>at</strong>e (18 cm 3 /mol) and <strong>the</strong> molar volume derived from <strong>the</strong><br />
estim<strong>at</strong>ed van der Waals volume of <strong>the</strong> w<strong>at</strong>er molecule (11.7<br />
Å 3 , correspond<strong>in</strong>g to a molar volume of 7.1 cm 3 /mol 23 ). In <strong>the</strong><br />
glassy carbohydr<strong>at</strong>e m<strong>at</strong>rix, w<strong>at</strong>er consequently exists <strong>in</strong> a<br />
strongly nonideal st<strong>at</strong>e, as is confirmed by a Zimm-Lundberg<br />
cluster<strong>in</strong>g analysis of <strong>the</strong> w<strong>at</strong>er sorption iso<strong>the</strong>rm (Figure 5b;<br />
Table 4).<br />
The density of <strong>the</strong> carbohydr<strong>at</strong>e m<strong>at</strong>rix, which <strong>in</strong>creases from<br />
1.51 g/cm 3 <strong>at</strong> a w ) 0 to 1.55 g/cm 3 <strong>at</strong> a w ) 0.54 <strong>in</strong> <strong>the</strong> glassy<br />
st<strong>at</strong>e, <strong>in</strong>itially cont<strong>in</strong>ues to rise <strong>in</strong> <strong>the</strong> same l<strong>in</strong>ear fashion <strong>in</strong><br />
<strong>the</strong> rubbery st<strong>at</strong>e (viz. <strong>the</strong> value of 1.56 g/cm 3 <strong>at</strong> a w ) 0.75;<br />
Figure 4). Consequently, <strong>the</strong> partial molar volume of w<strong>at</strong>er <strong>in</strong><br />
<strong>the</strong> <strong>in</strong>itial part of <strong>the</strong> rubbery st<strong>at</strong>e is about ∼9 cm 3 /mol, i.e.,<br />
close to its value <strong>in</strong> <strong>the</strong> glassy st<strong>at</strong>e. N<strong>at</strong>urally, <strong>at</strong> very high<br />
w<strong>at</strong>er contents, <strong>the</strong> density will start to decrease to f<strong>in</strong>ally reach<br />
<strong>the</strong> value of liquid w<strong>at</strong>er <strong>in</strong> <strong>the</strong> concentr<strong>at</strong>ion limit of zero