Millikan Oil Drop Experiment
Millikan Oil Drop Experiment
Millikan Oil Drop Experiment
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
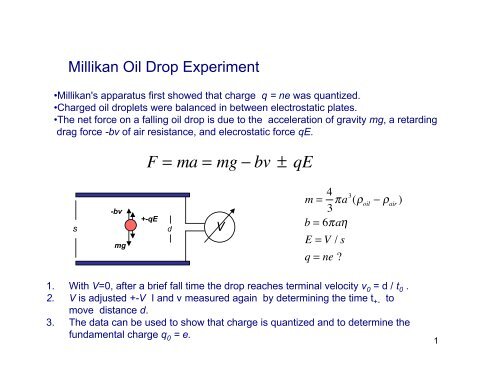
<strong>Millikan</strong> <strong>Oil</strong> <strong>Drop</strong> <strong>Experiment</strong><br />
•<strong>Millikan</strong>'s apparatus first showed that charge q = ne was quantized.<br />
•Charged oil droplets were balanced in between electrostatic plates.<br />
•The net force on a falling oil drop is due to the acceleration of gravity mg, a retarding<br />
drag force -bv of air resistance, and elecrostatic force qE.<br />
F = ma = mg ! bv!±!qE!!!!!!!!<br />
s<br />
-bv<br />
mg<br />
+-qE<br />
d<br />
V<br />
m = 4 3 !a3 (" oil<br />
# " air<br />
)!!!<br />
b = 6!a$<br />
E = V / s<br />
q = ne!?<br />
1. With V=0, after a brief fall time the drop reaches terminal velocity v 0 = d / t 0 .<br />
2. V is adjusted +-V I and v measured again by determining the time t +- to<br />
move distance d.<br />
3. The data can be used to show that charge is quantized and to determine the<br />
fundamental charge q 0 = e.<br />
1
Solving First order DE<br />
m dv<br />
dt<br />
!<br />
v<br />
#<br />
0<br />
v<br />
#<br />
0<br />
= F(v)!!!!!!!!!!!!!!!!!!!!!!!!!!! F(v) =!"(mg + bv)<br />
dv<br />
F(v) = 1 t<br />
m<br />
# dt<br />
0<br />
dv<br />
( mg = "b<br />
t<br />
b + v) m<br />
# dt<br />
0<br />
v<br />
ln( mg<br />
b + v) !!= "b<br />
0<br />
m !!t!!!!!!!!!!!!!!$!!!!!!v(t) =! % mg (<br />
&<br />
'<br />
b )<br />
* (1" e<br />
t >> 0!!!v 0<br />
=! mg<br />
b !!!terminal!!!velocity<br />
"b<br />
m !!t )<br />
v<br />
t<br />
v o<br />
2
<strong>Millikan</strong> <strong>Oil</strong> <strong>Drop</strong> <strong>Experiment</strong> (2)<br />
qE =!mg + b! d t !!!!!!!!Equation!of motion!after!ter minal!velocity!reached.!v 0<br />
= d t 0<br />
!!!!!(1)<br />
1<br />
t ±<br />
=<br />
!<br />
"<br />
#<br />
E $ !<br />
b!d %<br />
& q '<br />
mg $ ! ±V / s$<br />
"<br />
#<br />
b!d %<br />
& !!!=!!!<br />
"<br />
#<br />
b!d %<br />
& q +! 1 !!!!!!!!(!!!!! 1 ' 1 ! | V | /s$<br />
= 2!<br />
t 0<br />
t +<br />
t '<br />
"<br />
#<br />
b!d %<br />
& q!!!!!!!!!!!!!!(2)!!!<br />
1 !<br />
= '<br />
mg $<br />
t 0<br />
"<br />
#<br />
b!d %<br />
& =! 2 9<br />
a 2 () oil<br />
' ) air<br />
)<br />
g!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!(3)<br />
*!d<br />
1) See data in Table 1.1 for your calculations.<br />
2) Use Eq (3) t 0 to find the average drop radius a for each case. You<br />
will need this to find b in step 3).<br />
3) Use Eq (2) to find q for each trial (1-12).<br />
4) Assume fundamental charge e=q min .<br />
5) Determine n = q/q min and round off n to the nearest integer.<br />
6) Graph 1/t vs n (see Figure 1.4). How does this linear plot<br />
show that q is quantized?<br />
7) Determine the best value of the electron charge e = q min from the 12 data<br />
points (drop-1 and drop-2).<br />
8) Values of ρ−oil and ρ-air are in the text.<br />
3