Regulatory Aspects of Impurities in Biological Products - IIR
Regulatory Aspects of Impurities in Biological Products - IIR
Regulatory Aspects of Impurities in Biological Products - IIR
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
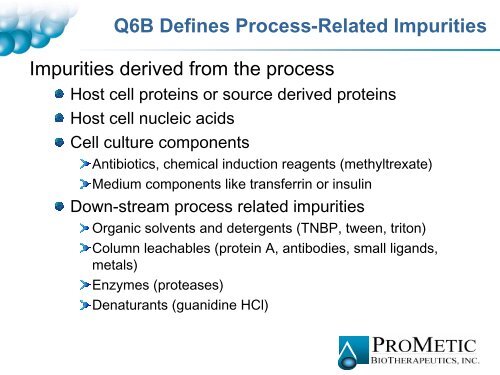
Q6B Def<strong>in</strong>es Process-Related <strong>Impurities</strong><br />
<strong>Impurities</strong> derived from the process<br />
Host cell prote<strong>in</strong>s or source derived prote<strong>in</strong>s<br />
Host cell nucleic acids<br />
Cell culture components<br />
Antibiotics, chemical <strong>in</strong>duction reagents (methyltrexate)<br />
Medium components like transferr<strong>in</strong> or <strong>in</strong>sul<strong>in</strong><br />
Down-stream process related impurities<br />
Organic solvents and detergents (TNBP, tween, triton)<br />
Column leachables (prote<strong>in</strong> A, antibodies, small ligands,<br />
metals)<br />
Enzymes (proteases)<br />
Denaturants (guanid<strong>in</strong>e HCl)