Download Issue PDF - Platinum Metals Review
Download Issue PDF - Platinum Metals Review
Download Issue PDF - Platinum Metals Review
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
doi:10.1595/147106709X481372<br />
•<strong>Platinum</strong> <strong>Metals</strong> Rev., 2010, 54, (1)•<br />
with Ru-2 in 2 h at an S:C ratio of 100 and a temperature<br />
of 40ºC (73, 74). With the azeotropic HCOOH-<br />
NEt 3 mixture as the reductant, the same catalyst<br />
required about 60 h to complete the reduction (97%<br />
ee) at 28ºC and an S:C ratio of 200 (26). Likewise,<br />
1-acetonaphthone was reduced to (R)-1-(1-naphthyl)-<br />
ethanol with Ru-2 by aqueous HCOONa in 98% conversion<br />
and 87% ee in 6 h at 40ºC and an S:C ratio of<br />
100; but in the azeotropic HCOOH-NEt 3 mixture with<br />
Ru-1, a conversion of only 71% was achieved in 30 h<br />
at 50ºC (120). In the aqueous phase ATH, there<br />
appears to be no correlation between the electronic<br />
properties of substituents and the enantioselectivity,<br />
as shown by the reduction of para-OMe- and<br />
para-CF 3 -acetophenone with Ru-2, which both gave<br />
~95% ee in 2 h.<br />
For practical applications, easy separation of the<br />
catalyst and product is necessary. The Ru-1 catalyst<br />
allows for this, due to its hydrophilic PEG chain,<br />
which retains the catalyst in the water during<br />
product extraction with, for example, diethyl ether. In<br />
the case of acetophenone reduction, we measured<br />
the leached Ru; inductively coupled plasma (ICP)<br />
analysis showed that only 0.4 mol% of Ru leached<br />
into the organic phase. Remarkably, the PEG-immobilised<br />
catalyst could be reused fourteen times with<br />
no loss in enantioselectivity, demonstrating its excellent<br />
recyclability and lifetime under aqueous conditions.<br />
When HCOOH-NEt 3 was used without water,<br />
the recycle experiments could not be carried out for<br />
more than two runs without the rates and ees being<br />
eroded.<br />
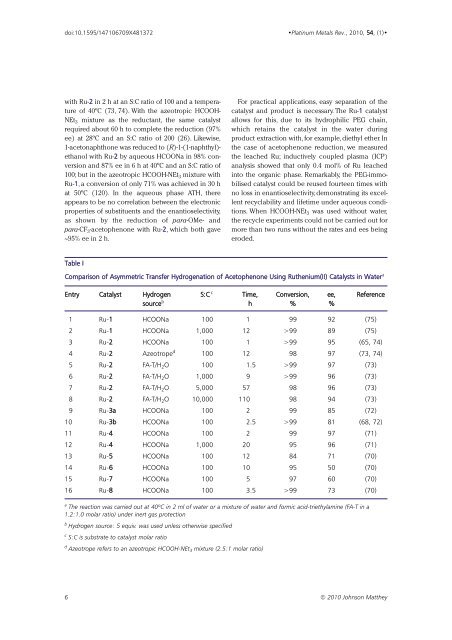
Table I<br />
Comparison of Asymmetric Transfer Hydrogenation of Acetophenone Using Ruthenium(II) Catalysts in Water a<br />
Entry Catalyst Hydrogen S:C c Time, Conversion, ee, Reference<br />
source b h % %<br />
1 Ru-1 HCOONa 100 1 99 92 (75)<br />
2 Ru-1 HCOONa 1,000 12 >99 89 (75)<br />
3 Ru-2 HCOONa 100 1 >99 95 (65, 74)<br />
4 Ru-2 Azeotrope d 100 12 98 97 (73, 74)<br />
5 Ru-2 FA-T/H 2 O 100 1.5 >99 97 (73)<br />
6 Ru-2 FA-T/H 2 O 1,000 9 >99 96 (73)<br />
7 Ru-2 FA-T/H 2 O 5,000 57 98 96 (73)<br />
8 Ru-2 FA-T/H 2 O 10,000 110 98 94 (73)<br />
9 Ru-3a HCOONa 100 2 99 85 (72)<br />
10 Ru-3b HCOONa 100 2.5 >99 81 (68, 72)<br />
11 Ru-4 HCOONa 100 2 99 97 (71)<br />
12 Ru-4 HCOONa 1,000 20 95 96 (71)<br />
13 Ru-5 HCOONa 100 12 84 71 (70)<br />
14 Ru-6 HCOONa 100 10 95 50 (70)<br />
15 Ru-7 HCOONa 100 5 97 60 (70)<br />
16 Ru-8 HCOONa 100 3.5 >99 73 (70)<br />
a The reaction was carried out at 40ºC in 2 ml of water or a mixture of water and formic acid-triethylamine (FA-T in a<br />
1.2:1.0 molar ratio) under inert gas protection<br />
b Hydrogen source: 5 equiv. was used unless otherwise specified<br />
c S:C is substrate to catalyst molar ratio<br />
d Azeotrope refers to an azeotropic HCOOH-NEt 3 mixture (2.5:1 molar ratio)<br />
6 © 2010 Johnson Matthey