Concentration
Concentration
Concentration
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
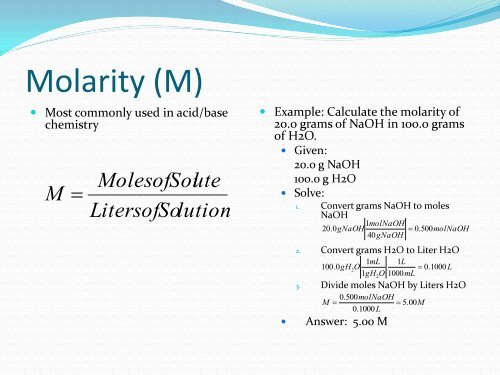
Molarity (M)<br />
• Most commonly used in acid/base<br />
chemistry<br />
M<br />
MolesofSolute<br />
LitersofSolution<br />
• Example: Calculate the molarity of<br />
20.0 grams of NaOH in 100.0 grams<br />
of H2O.<br />
• Given:<br />
20.0 g NaOH<br />
100.0 g H2O<br />
• Solve:<br />
1. Convert grams NaOH to moles<br />
NaOH<br />
1molNaOH<br />
20.0gNaOH 0. 500molNaOH<br />
40gNaOH<br />
2. Convert grams H2O to Liter H2O<br />
1mL<br />
1L<br />
100.0gH2O<br />
0. 1000 L<br />
1gH O 1000 mL<br />
3. Divide moles NaOH by Liters H2O<br />
0.500 molNaOH<br />
M 5. 00M<br />
0.1000 L<br />
• Answer: 5.00 M<br />
2