Unit 2- Measurements, Math, and the Mole
Unit 2- Measurements, Math, and the Mole
Unit 2- Measurements, Math, and the Mole
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
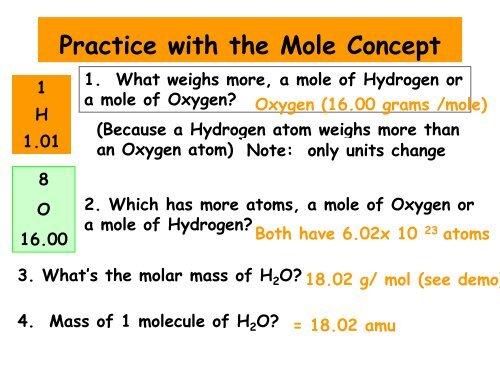
Practice with <strong>the</strong> <strong>Mole</strong> Concept<br />
1<br />
H<br />
1.01<br />
8<br />
O<br />
16.00<br />
1. What weighs more, a mole of Hydrogen or<br />
a mole of Oxygen? Oxygen (16.00 grams /mole)<br />
(Because a Hydrogen atom weighs more than<br />
an Oxygen atom) Note: only units change<br />
2. Which has more atoms, a mole of Oxygen or<br />
a mole of Hydrogen? Both have 6.02x 10<br />
23<br />
atoms<br />
3. What’s <strong>the</strong> molar mass of H 2 O? 18.02 g/ mol (see demo)<br />
4. Mass of 1 molecule of H 2 O? = 18.02 amu