The chemical behaviour of cyanide in the extraction of gold ... - saimm
The chemical behaviour of cyanide in the extraction of gold ... - saimm
The chemical behaviour of cyanide in the extraction of gold ... - saimm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
0,6<br />
0,5<br />
Carbon<br />
)C Og/l<br />
. 5g/1<br />
...25g/l<br />
.40 g/l<br />
0,6<br />
0,5<br />
pH<br />
~ 8,0<br />
. 9,0<br />
A 10,2<br />
10,9<br />
D 12,5<br />
014,0<br />
0,4<br />
0,4<br />
::::::<br />
bl)<br />
Z 0,3<br />
u t.s<br />
~<br />
0,2<br />
0,1<br />
Q)<br />
bl)<br />
t.s<br />
......<br />
'"§<br />
';::<br />
S<br />
';::<br />
Q)<br />
e-<br />
~ g<br />
-g<br />
-<br />
~<br />
'r;;<br />
t.s<br />
Q)<br />
u ....<br />
's. ;.,<br />
~<br />
::::::<br />
bl)<br />
Z 0,3<br />
u t.s<br />
~<br />
0,2<br />
0,1<br />
0<br />
0 10 20 30 40<br />
Time, h<br />
Conditions<br />
Volume <strong>of</strong> solution 500ml<br />
pH 10,2<br />
02 <strong>in</strong> solution 6,5 mg/l<br />
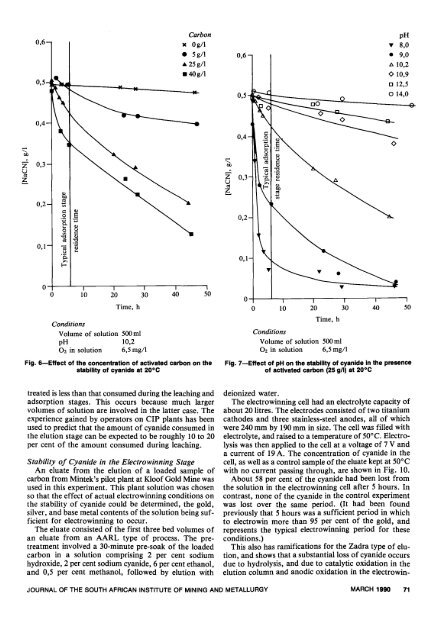
Fig. 6-Effect <strong>of</strong> <strong>the</strong> concentration <strong>of</strong> activated carbon on <strong>the</strong><br />
stability <strong>of</strong> <strong>cyanide</strong> at 20°C<br />
50<br />
0<br />
Fig. 7-Effect<br />
0 10 20 30 40 50<br />
Time,<br />
Conditions<br />
Volume <strong>of</strong> solution 500ml<br />
02 <strong>in</strong> solution 6,5 mg/l<br />
<strong>of</strong> pH on <strong>the</strong> stability <strong>of</strong> <strong>cyanide</strong> In <strong>the</strong> presence<br />
<strong>of</strong> activated carbon (25 g/l) at 20°C<br />
h<br />
~<br />
treated is less than that consumed dur<strong>in</strong>g <strong>the</strong> leach<strong>in</strong>g and<br />
adsorption stages. This occurs because much larger<br />
volumes <strong>of</strong> solution are <strong>in</strong>volved <strong>in</strong> <strong>the</strong> latter case. <strong>The</strong><br />
experience ga<strong>in</strong>ed by operators on CIF plants has been<br />
used to predict that <strong>the</strong> amount <strong>of</strong> <strong>cyanide</strong> consumed <strong>in</strong><br />
<strong>the</strong> elution stage can be expected to be roughly 10 to 20<br />
per cent <strong>of</strong> <strong>the</strong> amount consumed dur<strong>in</strong>g leach<strong>in</strong>g.<br />
Stability <strong>of</strong> Cyanide <strong>in</strong> <strong>the</strong> Electrow<strong>in</strong>n<strong>in</strong>g Stage<br />
An eluate from <strong>the</strong> elution <strong>of</strong> a loaded sample <strong>of</strong><br />
carbon from M<strong>in</strong>tek's pilot plant at Klo<strong>of</strong> Gold M<strong>in</strong>e was<br />
used <strong>in</strong> this experiment. This plant solution was chosen<br />
so that <strong>the</strong> effect <strong>of</strong> actual electrow<strong>in</strong>n<strong>in</strong>g conditions on<br />
<strong>the</strong> stability <strong>of</strong> <strong>cyanide</strong> could be determ<strong>in</strong>ed, <strong>the</strong> <strong>gold</strong>,<br />
silver, and base metal contents <strong>of</strong> <strong>the</strong> solution be<strong>in</strong>g sufficient<br />
for electrow<strong>in</strong>n<strong>in</strong>g to occur.<br />
<strong>The</strong> eluate consisted <strong>of</strong> <strong>the</strong> first three bed volumes <strong>of</strong><br />
an eluate from an AARL type <strong>of</strong> process. <strong>The</strong> pretreatment<br />
<strong>in</strong>volved a 30-m<strong>in</strong>ute pre-soak <strong>of</strong> <strong>the</strong> loaded<br />
carbon <strong>in</strong> a solution compris<strong>in</strong>g 2 per cent sodium<br />
hydroxide, 2 per cent sodium <strong>cyanide</strong>, 6 per cent ethanol,<br />
and 0,5 per cent methanol, followed by elution with<br />
deionized water.<br />
<strong>The</strong> electrow<strong>in</strong>n<strong>in</strong>g cell had an electrolyte capacity <strong>of</strong><br />
about 20 litres. <strong>The</strong> electrodes consisted <strong>of</strong> two titanium<br />
cathodes and three sta<strong>in</strong>less-steel anodes, all <strong>of</strong> which<br />
were 240 mm by 190 mm <strong>in</strong> size. <strong>The</strong> cell was filled with<br />
electrolyte, and raised to a temperature <strong>of</strong> 50°C. Electrolysis<br />
was <strong>the</strong>n applied to <strong>the</strong> cell at a voltage <strong>of</strong> 7 V and<br />
a current <strong>of</strong> 19 A. <strong>The</strong> concentration <strong>of</strong> <strong>cyanide</strong> <strong>in</strong> <strong>the</strong><br />
cell, as well as a control sample <strong>of</strong> <strong>the</strong> eluate kept at 50°C<br />
with no current pass<strong>in</strong>g through, are shown <strong>in</strong> Fig. 10.<br />
About 58 per cent <strong>of</strong> <strong>the</strong> <strong>cyanide</strong> had been lost from<br />
<strong>the</strong> solution <strong>in</strong> <strong>the</strong> electrow<strong>in</strong>n<strong>in</strong>g cell after 5 hours. In<br />
contrast, none <strong>of</strong> <strong>the</strong> <strong>cyanide</strong> <strong>in</strong> <strong>the</strong> control experiment<br />
was lost over <strong>the</strong> same period. (It had been found<br />
previously that 5 hours was a sufficient period <strong>in</strong> which<br />
to electrow<strong>in</strong> more than 95 per cent <strong>of</strong> <strong>the</strong> <strong>gold</strong>, and<br />
represents <strong>the</strong> typical electrow<strong>in</strong>n<strong>in</strong>g period for <strong>the</strong>se<br />
conditions.)<br />
This also has ramifications for <strong>the</strong> Zadra type <strong>of</strong> elution,<br />
and shows that a substantial loss <strong>of</strong> <strong>cyanide</strong> occurs<br />
due to hydrolysis, and due to catalytic oxidation <strong>in</strong> <strong>the</strong><br />
elution column and anodic oxidation <strong>in</strong> <strong>the</strong> electrow<strong>in</strong>-<br />
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY MARCH 1990 71