Development and Characterization of ... - Sciensage.info
Development and Characterization of ... - Sciensage.info
Development and Characterization of ... - Sciensage.info
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Swelling Index %w/w<br />
% Microcapsules retained after<br />
8th hour<br />
Santhosh Kumar Mankala et al, J Adv Scient Res, 2011; 2(4): 34-45 43<br />
Table 3: Drug content/Encapsulation Efficiency <strong>of</strong> formulations NSM 1 -NMM 6<br />
Formulation<br />
D:SA:P<br />
ratio<br />
Weight<br />
Taken<br />
(mg)<br />
Theoretical<br />
Drug content<br />
(mg)<br />
Practical<br />
Drug Content<br />
(mg)<br />
Encapsulation<br />
Efficiency (%)<br />
NSM 1<br />
2:2:1 100 40 36.55 ±2.254 91.375 ±5.126<br />
NSM 2<br />
2:3:1 100 33.33 30.98 ±1.975 93.878 ±4.356<br />
NSM 3<br />
2:4:1 100 28.57 25.36 ±1.991 90.571 ±4.198<br />
NMM 4<br />
2:2:1 100 40 34.58 ±2.321 86.45 ±6.124<br />
NMM 5<br />
2:3:1 100 33.33 30.24 ±1.012 91.636 ±2.448<br />
NMM 6<br />
2:4:1 100 28.57 22.65 ±3.165 80.892 ±7.275<br />
*Mean ± S.D (n=3)<br />
250<br />
200<br />
150<br />
110.54<br />
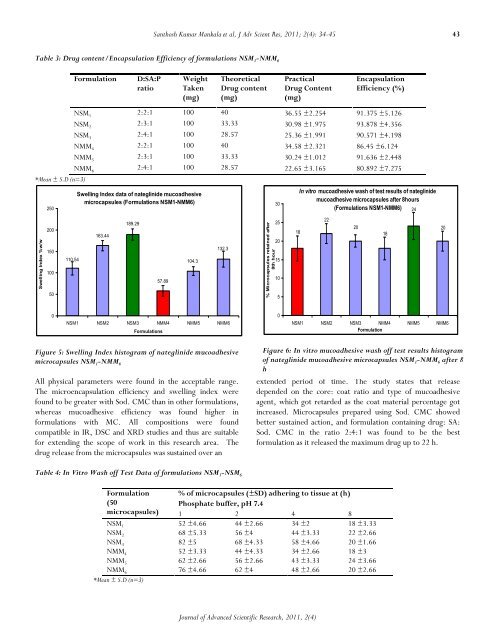
Swelling Index data <strong>of</strong> nateglinide mucoadhesive<br />
microcapsules (Formulations NSM1-NMM6)<br />
163.44<br />
189.29<br />
104.3<br />
132.3<br />
30<br />
25<br />
20<br />
15<br />
In vitro mucoadhesive wash <strong>of</strong> test results <strong>of</strong> nateglinide<br />
mucoadhesive microcapsules after 8hours<br />
(Formulations NSM1-NMM6) 24<br />
18<br />
22<br />
20<br />
18<br />
20<br />
100<br />
57.89<br />
10<br />
50<br />
5<br />
0<br />
NSM1 NSM2 NSM3 NMM4 NMM5 NMM6<br />
Formulations<br />
0<br />
NSM1 NSM2 NSM3 NMM4 NMM5 NMM6<br />
Formulation<br />
Figure 5: Swelling Index histogram <strong>of</strong> nateglinide mucoadhesive<br />
microcapsules NSM 1 -NMM 6<br />
All physical parameters were found in the acceptable range.<br />
The microencapsulation efficiency <strong>and</strong> swelling index were<br />
found to be greater with Sod. CMC than in other formulations,<br />
whereas mucoadhesive efficiency was found higher in<br />
formulations with MC. All compositions were found<br />
compatible in IR, DSC <strong>and</strong> XRD studies <strong>and</strong> thus are suitable<br />
for extending the scope <strong>of</strong> work in this research area. The<br />
drug release from the microcapsules was sustained over an<br />
Figure 6: In vitro mucoadhesive wash <strong>of</strong>f test results histogram<br />
<strong>of</strong> nateglinide mucoadhesive microcapsules NSM 1 -NMM 6 after 8<br />
h<br />
extended period <strong>of</strong> time. The study states that release<br />
depended on the core: coat ratio <strong>and</strong> type <strong>of</strong> mucoadhesive<br />
agent, which got retarded as the coat material percentage got<br />
increased. Microcapsules prepared using Sod. CMC showed<br />
better sustained action, <strong>and</strong> formulation containing drug: SA:<br />
Sod. CMC in the ratio 2:4:1 was found to be the best<br />
formulation as it released the maximum drug up to 22 h.<br />
Table 4: In Vitro Wash <strong>of</strong>f Test Data <strong>of</strong> formulations NSM 1 -NSM 6<br />
Formulation<br />
(50<br />
microcapsules)<br />
% <strong>of</strong> microcapsules (±SD) adhering to tissue at (h)<br />
Phosphate buffer, pH 7.4<br />
1 2 4 8<br />
NSM 1 52 ±4.66 44 ±2.66 34 ±2 18 ±3.33<br />
NSM 2 68 ±5.33 56 ±4 44 ±3.33 22 ±2.66<br />
NSM 3 82 ±5 68 ±4.33 58 ±4.66 20 ±1.66<br />
NMM 4 52 ±3.33 44 ±4.33 34 ±2.66 18 ±3<br />
NMM 5 62 ±2.66 56 ±2.66 43 ±3.33 24 ±3.66<br />
NMM 6 76 ±4.66 62 ±4 48 ±2.66 20 ±2.66<br />
*Mean ± S.D (n=3)<br />
Journal <strong>of</strong> Advanced Scientific Research, 2011, 2(4)