Unit 6: Periodic Table and Bonding - Mark Rosengarten
Unit 6: Periodic Table and Bonding - Mark Rosengarten
Unit 6: Periodic Table and Bonding - Mark Rosengarten
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
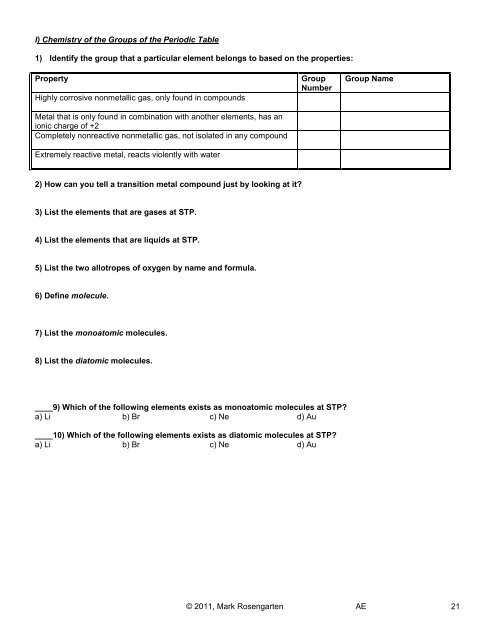
I) Chemistry of the Groups of the <strong>Periodic</strong> <strong>Table</strong><br />
1) Identify the group that a particular element belongs to based on the properties:<br />
Property<br />
Highly corrosive nonmetallic gas, only found in compounds<br />
Metal that is only found in combination with another elements, has an<br />
ionic charge of +2<br />
Completely nonreactive nonmetallic gas, not isolated in any compound<br />
Extremely reactive metal, reacts violently with water<br />
Group<br />
Number<br />
Group Name<br />
2) How can you tell a transition metal compound just by looking at it?<br />
3) List the elements that are gases at STP.<br />
4) List the elements that are liquids at STP.<br />
5) List the two allotropes of oxygen by name <strong>and</strong> formula.<br />
6) Define molecule.<br />
7) List the monoatomic molecules.<br />
8) List the diatomic molecules.<br />
____9) Which of the following elements exists as monoatomic molecules at STP?<br />
a) Li b) Br c) Ne d) Au<br />
____10) Which of the following elements exists as diatomic molecules at STP?<br />
a) Li b) Br c) Ne d) Au<br />
© 2011, <strong>Mark</strong> <strong>Rosengarten</strong> AE 21