Molarity - Revsworld
Molarity - Revsworld
Molarity - Revsworld
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
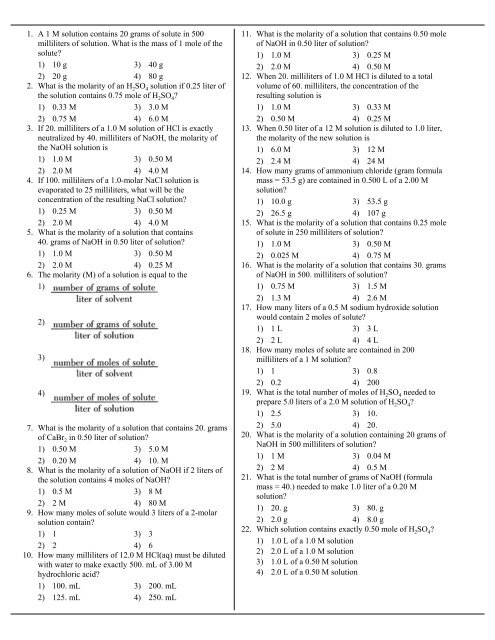
1. A 1 M solution contains 20 grams of solute in 500<br />
milliliters of solution. What is the mass of 1 mole of the<br />
solute?<br />
1) 10 g 3) 40 g<br />
2) 20 g 4) 80 g<br />
2. What is the molarity of an H 2 SO 4 solution if 0.25 liter of<br />
the solution contains 0.75 mole of H 2 SO 4 ?<br />
1) 0.33 M 3) 3.0 M<br />
2) 0.75 M 4) 6.0 M<br />
3. If 20. milliliters of a 1.0 M solution of HCl is exactly<br />
neutralized by 40. milliliters of NaOH, the molarity of<br />
the NaOH solution is<br />
1) 1.0 M 3) 0.50 M<br />
2) 2.0 M 4) 4.0 M<br />
4. If 100. milliliters of a 1.0-molar NaCl solution is<br />
evaporated to 25 milliliters, what will be the<br />
concentration of the resulting NaCl solution?<br />
1) 0.25 M 3) 0.50 M<br />
2) 2.0 M 4) 4.0 M<br />
5. What is the molarity of a solution that contains<br />
40. grams of NaOH in 0.50 liter of solution?<br />
1) 1.0 M 3) 0.50 M<br />
2) 2.0 M 4) 0.25 M<br />
6. The molarity (M) of a solution is equal to the<br />
1)<br />
2)<br />
3)<br />
4)<br />
7. What is the molarity of a solution that contains 20. grams<br />
of CaBr 2 in 0.50 liter of solution?<br />
1) 0.50 M 3) 5.0 M<br />
2) 0.20 M 4) 10. M<br />
8. What is the molarity of a solution of NaOH if 2 liters of<br />
the solution contains 4 moles of NaOH?<br />
1) 0.5 M 3) 8 M<br />
2) 2 M 4) 80 M<br />
9. How many moles of solute would 3 liters of a 2-molar<br />
solution contain?<br />
1) 1 3) 3<br />
2) 2 4) 6<br />
10. How many milliliters of 12.0 M HCl(aq) must be diluted<br />
with water to make exactly 500. mL of 3.00 M<br />
hydrochloric acid?<br />
1) 100. mL 3) 200. mL<br />
2) 125. mL 4) 250. mL<br />
11. What is the molarity of a solution that contains 0.50 mole<br />
of NaOH in 0.50 liter of solution?<br />
1) 1.0 M 3) 0.25 M<br />
2) 2.0 M 4) 0.50 M<br />
12. When 20. milliliters of 1.0 M HCl is diluted to a total<br />
volume of 60. milliliters, the concentration of the<br />
resulting solution is<br />
1) 1.0 M 3) 0.33 M<br />
2) 0.50 M 4) 0.25 M<br />
13. When 0.50 liter of a 12 M solution is diluted to 1.0 liter,<br />
the molarity of the new solution is<br />
1) 6.0 M 3) 12 M<br />
2) 2.4 M 4) 24 M<br />
14. How many grams of ammonium chloride (gram formula<br />
mass = 53.5 g) are contained in 0.500 L of a 2.00 M<br />
solution?<br />
1) 10.0 g 3) 53.5 g<br />
2) 26.5 g 4) 107 g<br />
15. What is the molarity of a solution that contains 0.25 mole<br />
of solute in 250 milliliters of solution?<br />
1) 1.0 M 3) 0.50 M<br />
2) 0.025 M 4) 0.75 M<br />
16. What is the molarity of a solution that contains 30. grams<br />
of NaOH in 500. milliliters of solution?<br />
1) 0.75 M 3) 1.5 M<br />
2) 1.3 M 4) 2.6 M<br />
17. How many liters of a 0.5 M sodium hydroxide solution<br />
would contain 2 moles of solute?<br />
1) 1 L 3) 3 L<br />
2) 2 L 4) 4 L<br />
18. How many moles of solute are contained in 200<br />
milliliters of a 1 M solution?<br />
1) 1 3) 0.8<br />
2) 0.2 4) 200<br />
19. What is the total number of moles of H 2 SO 4 needed to<br />
prepare 5.0 liters of a 2.0 M solution of H 2 SO 4 ?<br />
1) 2.5 3) 10.<br />
2) 5.0 4) 20.<br />
20. What is the molarity of a solution containing 20 grams of<br />
NaOH in 500 milliliters of solution?<br />
1) 1 M 3) 0.04 M<br />
2) 2 M 4) 0.5 M<br />
21. What is the total number of grams of NaOH (formula<br />
mass = 40.) needed to make 1.0 liter of a 0.20 M<br />
solution?<br />
1) 20. g 3) 80. g<br />
2) 2.0 g 4) 8.0 g<br />
22. Which solution contains exactly 0.50 mole of H 2 SO 4 ?<br />
1) 1.0 L of a 1.0 M solution<br />
2) 2.0 L of a 1.0 M solution<br />
3) 1.0 L of a 0.50 M solution<br />
4) 2.0 L of a 0.50 M solution
23. What is the total number of moles of solute contained in<br />
0.50 liter of 3.0 M HCl?<br />
1) 1.0 3) 3.0<br />
2) 1.5 4) 3.5<br />
24. What is the molarity of a solution of KNO 3 (molecular<br />
mass = 101) that contains 404 grams of KNO 3 in 2.00<br />
liters of solution?<br />
1) 1.00 3) 0.500<br />
2) 2.00 4) 4.00<br />
25. What is the total number of grams of HI in 0.500 liter of<br />
1.00 M HI?<br />
1) 1.00 g 3) 64.0 g<br />
2) 0.500 g 4) 128 g<br />
26. What is the concentration of a solution which contains 1<br />
mole of CaCl 2 dissolved in 2,000 milliliters of solution?<br />
1) 1 M 3) 0.5 M<br />
2) 2 M 4) 0.25 M<br />
27. If 0.50 liters of a 2.0M HCl is diluted with H 2 O to a<br />
volume of 1.0 liters, the molarity of the new solution will<br />
be<br />
1) 1.0 M 3) .25 M<br />
2) 2.0 M 4) .50 M<br />
28. What is the total number of grams of NaOH (formula<br />
mass = 40.) in 500. milliliters of a 1.0 M solution?<br />
1) 10. 3) 40.<br />
2) 20. 4) 80.<br />
29. What is the molarity of a solution containing 20. grams<br />
of NaOH in 0.50 liter of solution?<br />
1) 1.0 3) 0.50<br />
2) 2.0 4) 10.<br />
30. What is the concentration of a solution of 10. moles of<br />
copper (II) nitrate in 5.0 liters of solution?<br />
1) 0.50 M 3) 5.0 M<br />
2) 2.0 M 4) 10. M<br />
31. What is the molarity of a solution that contains 4 grams<br />
of NaOH in 500 milliliters of solution?<br />
[Formula mass of NaOH = 40.]<br />
1) 0.1 M 3) 0.2 M<br />
2) 2 M 4) 0.5 M<br />
32. What is the total number of moles of solute in 250<br />
milliliters of a 1.0 M solution of NaCl?<br />
1) 1.0 mole 3) 0.50 mole<br />
2) 0.25 mole 4) 42 moles<br />
33. A 20.-milliliter sample of 0.60 M HCl is diluted with<br />
water to a volume of 40. milliliters. What is the new<br />
concentration of the solution?<br />
1) 0.15 M 3) 0.30 M<br />
2) 0.60 M 4) 1.2 M<br />
34. What is the total number of moles of solute in 2.0 liters<br />
of 3.0 M NaOH?<br />
1) 1.0 mole 3) 3.0 moles<br />
2) 2.0 moles 4) 6.0 moles<br />
35. Which preparation produces a 2.0 M solution of C 6 H 12 O 6<br />
? [molecular mass = 180.0]<br />
1) 90.0 g of C 6 H 12 O 6 dissolved in 500.0 mL of solution<br />
2) 90.0 g of C 6 H 12 O 6 dissolved in 1000. mL of solution<br />
3) 180.0 g of C 6 H 12 O 6 dissolved in 500.0 mL of solution<br />
4) 180.0 g of C 6 H 12 O 6 dissolved in 1000. mL of solution<br />
36. How many moles of KNO 3 are required to make 0.50<br />
liter of a 2.0 M solution of KNO 3 ?<br />
1) 1.0 3) 0.50<br />
2) 2.0 4) 4.0<br />
37. How many grams of KOH are needed to prepare 250.<br />
milliliters of a 2.00 M solution of KOH<br />
(formula mass = 56.0)?<br />
1) 1.00 g 3) 28.0 g<br />
2) 2.00 g 4) 112 g<br />
38. What is the total number of grams of KCl (formula mass<br />
= 74.6) in 1.00 liter of 0.200 molar solution?<br />
1) 7.46 g 3) 22.4 g<br />
2) 14.9 g 4) 29.8 g<br />
39. What is the total number of grams of NaI(s) needed to<br />
make 1.0 liter of a 0.010 M solution?<br />
1) 0.015 3) 1.5<br />
2) 0.15 4) 15
Answer Key<br />
1. 3<br />
2. 3<br />
3. 3<br />
4. 4<br />
5. 2<br />
6. 4<br />
7. 2<br />
8. 2<br />
9. 4<br />
10. 2<br />
30. 2<br />
31. 3<br />
32. 2<br />
33. 3<br />
34. 4<br />
35. 3<br />
36. 1<br />
37. 3<br />
38. 2<br />
39. 3<br />
11. 1<br />
12. 3<br />
13. 1<br />
14. 3<br />
15. 1<br />
16. 3<br />
17. 4<br />
18. 2<br />
19. 3<br />
20. 1<br />
21. 4<br />
22. 3<br />
23. 2<br />
24. 2<br />
25. 3<br />
26. 3<br />
27. 1<br />
28. 2<br />
29. 1