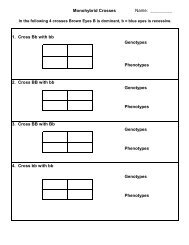
PS-3.5 - Solubility of Ammonium Chloride Lab
PS-3.5 - Solubility of Ammonium Chloride Lab
PS-3.5 - Solubility of Ammonium Chloride Lab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Solubility</strong> <strong>of</strong> <strong>Ammonium</strong> <strong>Chloride</strong><br />
Problem: How does surface area and temperature affect solubility?<br />
Hypothesis: _____________________________________________________________________________<br />
__________________________________________________________________________________________<br />
Experiment:<br />
Independent Variable: _________________________________________________________________<br />
Dependent Variable: ___________________________________________________________________<br />
Controls: _______________________________________________________________________________<br />
Safety:<br />
1. _________________________________________________________________________________<br />
2. _________________________________________________________________________________<br />
Materials:<br />
♦ 250 mL beaker<br />
♦ Thermometer<br />
♦ Hot plate<br />
Experiment:<br />
1. Put 50 mL <strong>of</strong> room temperature water into a clean beaker.<br />
♦ <strong>Ammonium</strong> chloride<br />
♦ Stir stick<br />
2. Use a thermometer to measure the temperature <strong>of</strong> water. Record this temperature in your<br />
Data Table. DO NOT LEAVE THE THERMOMETER IN THE BEAKER.<br />
3. Add one spoonful <strong>of</strong> ammonium chloride to the water in the beaker. Stir vigorously until<br />
no more <strong>of</strong> the solid will dissolve. Make sure you allow enough time for the solid to<br />
dissolve. Be careful handling ammonium chloride and its solutions, which are poisonous.<br />
4. Repeat step 3 until no more ammonium chloride will dissolve. Note the number <strong>of</strong><br />
spoonfuls <strong>of</strong> ammonium chloride that you added. Be sure to allow enough time for each<br />
spoonful <strong>of</strong> ammonium chloride to dissolve before adding more. Record the number <strong>of</strong><br />
spoonfuls that dissolved in your data table.<br />
5. Place the beaker <strong>of</strong> ammonium chloride solution on a hot plate. Place the thermometer in<br />
the beaker. Turn the hot plate to the medium-low setting and warm the solution to 40°C.<br />
6. Remove the thermometer from the beaker. Add more spoonfuls <strong>of</strong> ammonium chloride to<br />
the beaker. Stir the solution and use the thermometer to check the temperature <strong>of</strong> the<br />
solution after you add each spoonful. Note the total number <strong>of</strong> spoonfuls <strong>of</strong> ammonium<br />
chloride that you added. Be sure to allow enough time for each spoonful <strong>of</strong> ammonium<br />
chloride to dissolve before adding more. Use the heat setting <strong>of</strong> the hot plate to keep the<br />
temperature constant as you add ammonium chloride.<br />
7. Record the total number <strong>of</strong> spoonfuls that have dissolved.
8. Turn up the hot plate to warm the solution to 60°C. Then, repeat steps 6 and 7 with the<br />
solution at this temperature.<br />
9. Turn up the hot plate to warm the solution to 80°C. Then, repeat steps 6 and 7 with the<br />
solution at this temperature.<br />
10. When you have recorded all <strong>of</strong> your data, turn <strong>of</strong>f the hot plate and allow the solution to<br />
cool. Rinse the solutions down the drain. Let the water run continuously for about 1<br />
minute.<br />
11. On a sheet <strong>of</strong> paper, make a graph <strong>of</strong> the solubility <strong>of</strong> ammonium chloride versus<br />
temperature. Plot temperature on the horizontal x-axis and solubility (in spoonfuls per 50<br />
mL water) on the vertical axis.<br />
Observations:<br />
Temperature<br />
(°C)<br />
18-20<br />
Number <strong>of</strong> Spoonfuls <strong>of</strong> <strong>Ammonium</strong> <strong>Chloride</strong><br />
Dissolved<br />
40<br />
60<br />
80<br />
Include the following questions in your discussion and don’t forget a conclusion:<br />
1. Create a graph <strong>of</strong> the above information.<br />
2. Predict what effect, if any, you think the size <strong>of</strong> salt grains would have on their solubility.<br />
Explain your answer.<br />
3. Based on your graph and your observations what do you think the solubility <strong>of</strong> ammonium<br />
chloride (in spoonfuls per 50 mL water) would be at 10°C? At 70°C? At 100°C?