You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>20</strong>. <strong>Metals</strong> <strong>and</strong> <strong>Their</strong> <strong>Compounds</strong><br />
The two categories of elements, metals <strong>and</strong> nonmetals,<br />
are so different that most elements can be easily<br />
distinguished as either a metal or a nonmetal.<br />
<strong>Compounds</strong> can also be classified, although there are<br />
three categories rather than two: combinations of metal<br />
with metal, metal with nonmetal, <strong>and</strong> nonmetal with<br />
nonmetal. Each class has a distinctive set of characteristics.<br />
The combination of two metals produces an alloy<br />
that is difficult to distinguish from a pure metal because<br />
its characteristics are also metallic. A metal combined<br />
with a nonmetal yields a salt. The majority of such<br />
combinations are difficult to distinguish visually from<br />
table salt, but some are colored <strong>and</strong> nearly all are toxic.<br />
The combination of two nonmetals produces a material—usually<br />
a transparent gas—that is difficult to distinguish<br />
from a pure nonmetallic element.<br />
These categories show regularities yet to be<br />
explained: Why are salts so different from pure elements?<br />
Why can most metals be melted together in any<br />
proportion <strong>and</strong> then be solidified into a homogeneous<br />
alloy such that none of either starting metal is left as<br />
excess? Why does a compound composed of a metal<br />
<strong>and</strong> a nonmetal or of a nonmetal <strong>and</strong> another nonmetal<br />
nearly always leave some of one reactant in excess,<br />
unchanged at the end of the reaction? This chapter will<br />
begin to answer these questions.<br />
Pure <strong>Metals</strong> <strong>and</strong> Alloys<br />
A metal atom has at least one loosely held electron<br />
in a large outer orbital. The atom also has room for<br />
another electron. These facts account for the properties<br />
of metals in the following way. In a solid metal the<br />
atoms are close enough together that the orbitals of the<br />
loosely held valence electrons try to spread out into the<br />
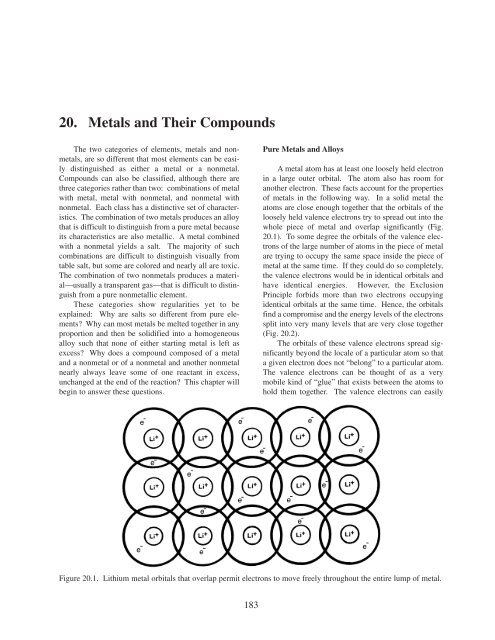
whole piece of metal <strong>and</strong> overlap significantly (Fig.<br />
<strong>20</strong>.1). To some degree the orbitals of the valence electrons<br />
of the large number of atoms in the piece of metal<br />
are trying to occupy the same space inside the piece of<br />
metal at the same time. If they could do so completely,<br />
the valence electrons would be in identical orbitals <strong>and</strong><br />
have identical energies. However, the Exclusion<br />
Principle forbids more than two electrons occupying<br />
identical orbitals at the same time. Hence, the orbitals<br />
find a compromise <strong>and</strong> the energy levels of the electrons<br />
split into very many levels that are very close together<br />
(Fig. <strong>20</strong>.2).<br />
The orbitals of these valence electrons spread significantly<br />
beyond the locale of a particular atom so that<br />
a given electron does not “belong” to a particular atom.<br />
The valence electrons can be thought of as a very<br />
mobile kind of “glue” that exists between the atoms to<br />
hold them together. The valence electrons can easily<br />
Figure <strong>20</strong>.1. Lithium metal orbitals that overlap permit electrons to move freely throughout the entire lump of metal.<br />
183
Figure <strong>20</strong>.2. Lithium orbital energies split <strong>and</strong> form many energy levels that are very close together as more <strong>and</strong> more<br />
atoms are added.<br />
Figure <strong>20</strong>.3. Malleability of a metal. (a) Forces applied to a group of metal atoms. (b) The solid deforms, but will<br />
spring back to its original shape if the force is removed. (c) The solid is deformed permanently by layers sliding to new<br />
positions.<br />
drift from one orbital to another through the overlapping<br />
regions to any part of the lump of metal.<br />
The drifting electrons give metals their unique<br />
properties, the most common of which are discussed<br />
below.<br />
1. Electrical conductivity. If electrons are<br />
forced into one end of a piece of metal, they<br />
push other mobile electrons out the other end.<br />
This is the mechanism by which wires carry<br />
electricity <strong>and</strong> is called electrical conductivity.<br />
2. Metallic luster. The closely spaced energy<br />
levels shown in Figure <strong>20</strong>.2 permit metals to<br />
absorb virtually every visible wavelength of<br />
light <strong>and</strong> many invisible wavelengths. A photon<br />
of almost any energy can find an energy<br />
gap that just fits. However, the atoms do not<br />
keep the photons, but immediately radiate<br />
them back (reflection). If all of the visible<br />
light is reflected, the metal appears silver, or if<br />
it is roughened, it appears white. If some of<br />
the violet <strong>and</strong> blue light is permanently<br />
absorbed, the metal appears gold or copper<br />
colored. High reflectivity in at least a part of<br />
the visible spectrum confers metallic luster.<br />
3. Malleability. Things like glass shatter when<br />
hit with a hammer, but most metals simply<br />
bend or flatten, particularly if they are hot. For<br />
example, gold can be pounded thinner than<br />
paper (to about a millionth of a centimeter<br />
thickness). This characteristic response to<br />
force is termed malleability. On the microscopic<br />
level, the metal nuclei must slide past<br />
each other into new positions without ever<br />
substantially weakening the metallic bond.<br />
The sea of mobile electrons flows wherever it<br />
is needed to keep the bonds strong during<br />
bending <strong>and</strong> flattening operations (Fig. <strong>20</strong>.3).<br />
4. Thermal conductivity. If thermal energy is<br />
added to a piece of metal, the kinetic energy of<br />
the electrons is increased. The electrons move<br />
rapidly through the metal distributing this<br />
kinetic energy throughout by collisions. Thus,<br />
the whole piece of metal becomes hot much<br />
sooner than an equivalent piece of nonmetal.<br />
This transport of thermal energy is termed<br />
thermal conductivity.<br />
5. Chemical reactivity. The mobile electrons<br />
are easy prey for electron-hungry atoms such<br />
as oxygen, so most metals react easily to form<br />
oxides or other compounds with nonmetals. In<br />
fact, only a few metals (such as gold, silver,<br />
184
<strong>and</strong> copper) are found uncombined in nature.<br />
The others have reacted with oxygen or other<br />
nonmetals <strong>and</strong> are found as ores.<br />
6. Alloy formation. In metals, the sea of electrons<br />
accommodates one type of metal atom<br />
about as well as another, particularly if their<br />
sizes <strong>and</strong> orbital energy levels are similar. This<br />
means that metals near one another (especially<br />
vertically) in the Periodic Table should be able<br />
to substitute for one another in solid metals.<br />
Many pairs of metals permit substitution for<br />
one another in all proportions to form alloys<br />
simply by melting the two together. Some<br />
properties of the resulting alloy, such as density,<br />
are intermediate between those of the two<br />
components. Other properties can turn out<br />
quite different than one might expect from the<br />
original components. Some metals, that are far<br />
apart in the Periodic Table, will permit only<br />
limited substitution.<br />
Science attempts to explain these characteristics<br />
with one simple concept: Mobile electrons are the basis<br />
of a metal’s electrical conductivity, metallic luster, malleability,<br />
thermal conductivity, <strong>and</strong> chemical reactivity.<br />
Oxidation States<br />
According to Figure 17.7, metals have one or more<br />
electrons that are easily removed because they are near<br />
the top of the energy well. These elements are generally<br />
to the left in the Periodic Table. In contrast, nonmetals<br />
have vacancies for electrons deep in the well.<br />
<strong>Metals</strong> often give one or more electrons to a nonmetal,<br />
thus forming a positive metal ion <strong>and</strong> a negative nonmetal<br />
ion. In forming the ions, the orbitals of the<br />
valence electrons transfer from the metal atom to center<br />
on the nonmetal atom. These oppositely charged ions<br />
are then electrically attracted <strong>and</strong> become attached to<br />
one another through what is known as an ionic bond.<br />
The oxidation state of an atom in a compound is a<br />
quantitative indication of the number of electrons that<br />
the atom has lost or gained in forming the ion or chemical<br />
bond. An atom that has lost an electron is said to be<br />
in the +1 oxidation state; an example is Li 1+ . An atom<br />
that has lost two electrons is in the +2 oxidation state;<br />
an example is Be 2+ . Similarly, there can be negative<br />
oxidation states for atoms that acquire extra electrons;<br />
examples are –1 for Cl 1– <strong>and</strong> –2 for O 2– .<br />
The important thing to note is that stable chemical<br />
bonds will form if doing so lowers the overall energies<br />
Figure <strong>20</strong>.4. Common oxidation states of the elements.<br />
185
of the valence electrons by putting them lower in energy<br />
wells. The filled shells of the noble gases (helium,<br />
neon, argon, etc.) are particularly low energy arrangements<br />
of the electrons. These elements do not react<br />
with other elements because the reaction does not provide<br />
a means to an overall lowering of the electron energies.<br />
The effective oxidation state of the noble gases is<br />
identically zero. Many ions achieve this “noble gas<br />
electron configuration,” but of course no change has<br />
been made to the nucleus.<br />
In general, metallic elements near the left of the<br />
Periodic Table assume positive oxidation states in<br />
chemical reactions. Nonmetallic elements near the right<br />
side of the Periodic Table assume negative oxidation<br />
states. Near the middle of the Periodic Table things are<br />
somewhat ambiguous. Will an element like carbon<br />
(which has four valence electrons) lose its four valence<br />
electrons (like a metal), or gain four to fill its shell (like<br />
a nonmetal)? The answer depends on the specific compound<br />
that is being formed. An element in the middle,<br />
such as carbon, will do whichever best lowers its electron<br />
energy. Thus, for elements near the middle of the<br />
chart there is a tendency for atoms to assume different<br />
oxidation states in different chemical reactions, depending<br />
on the particular compound which is being formed.<br />
Although many elements can have more than one<br />
oxidation state (depending on the compound), the primary<br />
(likely) oxidation state is related to the atom’s position<br />
in the Periodic Table. All of the elements in column<br />
1 (labeled IA in the Periodic Table) will have an oxidation<br />
state of +1 in ionic compounds. Elements in IIA<br />
have +2 oxidation states. What would you predict for<br />
column IIIA? Check your answer in Figure <strong>20</strong>.4.<br />
Elements in columns VIA <strong>and</strong> VIIA of the Periodic<br />
Table tend to gain electrons to fill their valence shells<br />
<strong>and</strong> can be predicted with confidence to have primary<br />
oxidation states of –2 <strong>and</strong> –1, respectively. Elements in<br />
these columns sometimes have additional possible oxidation<br />
states in compounds. See sulfur <strong>and</strong> chlorine in<br />
Figure <strong>20</strong>.4, for example.<br />
Elements in the B columns of the Periodic Table are<br />
also prone to having more than one possible oxidation<br />
state. However, we can use the additional information<br />
that these elements are all metals (<strong>and</strong>, therefore, tend to<br />
be electron donors) to predict that at least in some compounds<br />
these elements will have positive oxidation<br />
states that correspond directly to the number of valence<br />
electrons indicated by their column number. For example,<br />
chromium ( 24 Cr) in column VIB will in at least<br />
some cases have an oxidation state of +6. The rule is<br />
even less precise for the elements in the centermost<br />
columns of the Periodic Table (labeled VIII [neither A<br />
nor B]). Here we can only predict that, as metals, the<br />
elements will have positive oxidation states, but we<br />
must rely on experimental analysis of the individual<br />
compounds to determine the precise oxidation states of<br />
the constituent elements.<br />
Why do the regularities exist among the elements?<br />
They are based on ionization energies whose precise values<br />
can be traced back to the Wave Model <strong>and</strong> the<br />
Exclusion Principle. If only one electron in an atom has<br />
a low ionization energy, the oxidation state will be +1. If<br />
two electrons have low ionization energies, the oxidation<br />
state will be +2, <strong>and</strong> so on. If there are no electrons with<br />
low ionization energies, but there is a vacancy in an<br />
orbital deep in the well, the atom will try to fill the vacancy<br />
with an electron. This results in a –1 oxidation state.<br />
<strong>Compounds</strong> Between <strong>Metals</strong> <strong>and</strong> Nonmetals<br />
Imagine a metallic sodium ion, Na + , <strong>and</strong> a nonmetallic<br />
chloride ion, Cl – , that could form an ionic bond. But<br />
why should the pairing stop there? Could the Na + attract<br />
another negative ion <strong>and</strong> the Cl – attract another positive<br />
ion? Yes. Long chains of alternating Na + <strong>and</strong> Cl – ions<br />
form a sheet like a checkerboard (Fig. <strong>20</strong>.5), <strong>and</strong> finally<br />
the checkerboards stack so that positive ions are always<br />
above negative ions <strong>and</strong> vice versa. The result is a crystal<br />
of salt based on the electrical attraction of the ions.<br />
Figure <strong>20</strong>.5. Drawing of a small part of a single layer<br />
of a NaCl (sodium chloride) crystal.<br />
A salt is much different than a metal. Ionic bonding<br />
accounts for the characteristics of salts discussed<br />
below.<br />
1. Electrically nonconducting. The electrons in<br />
both ions of a salt are all in stable, closed<br />
shells, <strong>and</strong> are difficult to remove from their<br />
respective ions. Because there are no mobile<br />
electrons, solid salts have no way to conduct<br />
electricity. However, if the salt is melted,<br />
whole ions may move about <strong>and</strong> conduct electricity,<br />
but they are so big <strong>and</strong> awkward they<br />
are not nearly as conductive as the electrons in<br />
a metal. Also, if the salt is dissolved in a liquid<br />
like water, the ions separate <strong>and</strong> are able to<br />
186
conduct electricity, but in an inefficient manner.<br />
Materials that conduct electricity when<br />
dissolved in water are called electrolytes.<br />
2. Transparent. The energy levels of most salts<br />
are spaced widely apart, so most salts will<br />
absorbs only photons with high energies. Such<br />
high-energy photons are in the invisible, ultraviolet<br />
part of the spectrum. Visible light is not<br />
absorbed by most salts—it passes through <strong>and</strong><br />
the salts appear transparent. However, most of<br />
the salts of metals in columns IIIB through IIB<br />
absorb some visible light weakly; this gives<br />
them pale colors. There are also a few highly<br />
colored salts like potassium dichromate; most<br />
of them are composed of more than two elements<br />
(e.g., K 2 Cr 2 O 7 ). If a transparent salt<br />
crystal is ground to powder, the small crystals<br />
bend a light ray many times before it emerges<br />
from the powder. This means light will no<br />
longer pass straight through as it did in a single<br />
crystal, so light coming in from the side may<br />
bend <strong>and</strong> come out the front. The net result is<br />
that the powder appears white, even though it<br />
is basically transparent or slightly colored. A<br />
block of salt with many cracks or bubbles in it<br />
will also appear white. Many otherwise transparent<br />
minerals in nature appear opaque <strong>and</strong><br />
white for this reason.<br />
3. Brittle. Salt crystals break <strong>and</strong> shatter when<br />
hit with a hammer—they do not bend or flatten.<br />
Imagine sliding one layer of a NaCl crystal<br />
over the underlying layer (Fig. <strong>20</strong>.6). When<br />
one sliding layer moves a short distance, all of<br />
the positive ions will be directly over other<br />
positive ions, <strong>and</strong> likewise for the negative<br />
ions. The repulsion is more than enough to<br />
force the two layers apart, which breaks<br />
(cleaves) the crystal. Brittleness, however,<br />
does not always dominate. Glass, most rocks,<br />
<strong>and</strong> objects made of fired clay can be considered<br />
super-cooled liquids, because under<br />
mild stress, only a few atoms at a time move<br />
<strong>and</strong> no breakage occurs. Over a long time<br />
many atoms will have moved, <strong>and</strong> the object<br />
will have a different shape. Thick rock layers<br />
bend, fold, <strong>and</strong> take on some surprisingly contorted<br />
shapes if they are not pushed too rapidly.<br />
If they cannot adapt quickly enough, they<br />
snap <strong>and</strong> cause earthquakes.<br />
Formulas <strong>and</strong> Names of Salts<br />
The charge in a crystal must be balanced (same<br />
total positive as negative charge). Even a relatively<br />
Figure <strong>20</strong>.6. Force applied to layers of ions in a salt<br />
crystal will slide ions with the same charge over one<br />
another <strong>and</strong> split the crystal.<br />
small excess charge would cause a spark to jump from<br />
the crystal. This implies that there are nearly the same<br />
number of Na + as Cl – ions in a crystal of table salt. We<br />
can use subscripts to indicate how many ions there are<br />
in each crystal. For example, a small crystal containing<br />
70 of each type of ion would be designated Na 70 Cl 70 .<br />
Because the proportion is always one-to-one, it is customary<br />
to write the simplest formula, NaCl. Other oneto-one<br />
compounds are LiF <strong>and</strong> CaO. Note that the<br />
metal is written first, the nonmetal last.<br />
Now consider Mg 2+ ions coming together with Cl –<br />
ions to form a crystal. Two Cl – ions are required to neutralize<br />
the +2 charge on the magnesium ion. The proportion<br />
is now one magnesium to two chlorides, <strong>and</strong> the<br />
formula is MgCl 2 . By knowing the oxidation numbers,<br />
we can determine the proper proportions <strong>and</strong> the proper<br />
subscripts in the formulas of compounds composed of a<br />
metal <strong>and</strong> a nonmetal. The pivotal concept is the cancellation<br />
of charges.<br />
You should be cautioned that this system works<br />
best for the combination of a metal with a nonmetal. It<br />
predicts just one compound—there may be others. If<br />
the oxidation states are known, the rule works for nonmetals<br />
also. For example, nitrogen in the unusual oxidation<br />
state of +4 can combine with oxygen in its –2<br />
oxidation state. The resulting compound of two nonmetals<br />
has the formula NO 2 . However, it is technically<br />
incorrect to discuss such compounds in terms of oxida-<br />
187
tion “states” that imply that one of the atoms has entirely<br />
lost one or more electrons <strong>and</strong> the other atom has<br />
taken full possession of one or more electrons. Ions will<br />
not be formed completely by elements near one another<br />
in the Periodic Table. The smaller nonmetal atom will<br />
pull harder on the electrons than the larger metal atom<br />
does, but the electrons will not leave entirely.<br />
Nevertheless, the pretense that they leave helps us write<br />
down the correct formula, so we keep the concept but<br />
change the name to oxidation number to remind ourselves<br />
that we may not be dealing with completely<br />
formed ions.<br />
In naming these salts, the metal is named first followed<br />
by the nonmetal with its last syllable changed to<br />
-ide: NaCl is sodium chloride, Li 2 O is lithium oxide,<br />
<strong>and</strong> Al 2 S 3 is aluminum sulfide.<br />
The elements chosen for the preceding examples<br />
usually can have only one oxidation number. Many<br />
other elements have at least two common oxidation<br />
numbers; the name must indicate which is the oxidation<br />
number of interest. One way to do this is to use Roman<br />
numerals in parentheses to indicate the oxidation state<br />
of the metal. For example, FeO would be named<br />
iron(II) oxide, <strong>and</strong> Fe 2 O 3 would be named iron(III)<br />
oxide.<br />
When two nonmetals are combined, it is not always<br />
clear from the oxidation numbers how many atoms are<br />
in the molecule, so the number of atoms of each element<br />
in the molecule is mentioned using the following prefixes:<br />
mono- for one, di- for two, tri- for three, <strong>and</strong><br />
tetra- for four. (Other prefixes are used for higher numbers.)<br />
Some examples are CO, carbon monoxide; CO 2 ,<br />
carbon dioxide; <strong>and</strong> N 2 O 3 , dinitrogen trioxide.<br />
Summary<br />
Elements can be divided into two categories: metals<br />
<strong>and</strong> nonmetals. In turn we can divide the types of<br />
chemical bonds into three categories: metals with metals<br />
(metallic bond), metals with nonmetals (ionic bond),<br />
<strong>and</strong> nonmetals with nonmetals (covalent bond). A distinctive<br />
class of physical characteristics is associated<br />
with each type of bond.<br />
In a solid metal the atoms are so close together that<br />
the orbits of the valence electrons overlap. Because the<br />
outer electrons are so loosely held, they can easily drift<br />
from one orbital to another through the overlapping<br />
regions to any part of the lump of metal. The freedom<br />
of the valence electrons to drift results in the following<br />
properties for metals bound by the metallic bond: good<br />
electrical conductivity, metallic luster, malleability,<br />
good thermal conductivity, high chemical reactivity,<br />
<strong>and</strong> readiness to form alloys.<br />
It is energetically favorable in metal-nonmetal<br />
reactions for the metal to give up valence electrons to<br />
the nonmetal, thus forming electrically charged ions.<br />
Electrically charged ions with opposite charges are then<br />
attracted to one another <strong>and</strong> become attached to one<br />
another in an ionic bond. Atoms that have lost electrons<br />
in ionic bonds are said to be in positive oxidation<br />
states. Atoms that have gained electrons in ionic bonds<br />
are said to be in negative oxidation states. The oxidation<br />
states are further characterized by the number of<br />
electrons gained or lost: –1 (for atoms that have gained<br />
one electron), +2 (for atoms that have lost two electrons),<br />
etc.<br />
Ionic compounds of a metal <strong>and</strong> a nonmetal are<br />
called salts. Salts are generally crystalline solids, transparent,<br />
brittle, white in color (although occasionally<br />
colored), electrically nonconducting as a solid, but conducting<br />
in water solutions or in melted form. These<br />
characteristics can be easily understood in terms of the<br />
nature of the ionic bond.<br />
Bonding between nonmetals (covalent bond) will<br />
be described in the next chapter.<br />
STUDY GUIDE<br />
<strong>Chapter</strong> <strong>20</strong>: <strong>Metals</strong> <strong>and</strong> <strong>Their</strong> <strong>Compounds</strong><br />
A. FUNDAMENTAL PRINCIPLES: No new fundamental<br />
principles.<br />
B MODELS, IDEAS, QUESTIONS, OR APPLICA-<br />
TIONS<br />
1. What useful groups are compounds often divided<br />
into?<br />
2. Under what conditions do atoms form metallic<br />
bonds?<br />
3. What are the properties of compounds held in<br />
metallic bonds <strong>and</strong> why do these compounds have<br />
such properties?<br />
4. Under what conditions do atoms form ionic bonds?<br />
5. What are the simplest rules needed to determine the<br />
primary oxidation states of different atoms?<br />
6. What are the properties of compounds held in ionic<br />
bonds, <strong>and</strong> why do these compounds have such<br />
properties?<br />
7. What procedure leads to the correct chemical formula<br />
for reactants formed in reactions involving<br />
compounds held together in ionic bonds?<br />
C. GLOSSARY<br />
1. Alloys: Alloys are mixtures of metals. <strong>Metals</strong><br />
readily form alloys within certain limits.<br />
2. Brittleness: A characteristic of ionic substances,<br />
such as salts, that readily shatter when struck a<br />
sharp blow.<br />
3. Electrical Conductivity: A measure of the degree<br />
to which a substance conducts an electrical current.<br />
<strong>Metals</strong> have a high electrical conductivity.<br />
4. Electrical Nonconductivity: A characterization of<br />
ionic substances, such as salts, that do not readily<br />
188
conduct electricity.<br />
5. Ionic Bond: The chemical bond that binds a<br />
metallic ion to a nonmetallic ion by electrical<br />
attraction.<br />
6. Ions: A charged object formed when an atom or<br />
molecule loses or gains electrons.<br />
7. Malleability: The characteristic of substances that<br />
allows them to be worked into desirable shapes or<br />
drawn out into wires. <strong>Metals</strong> are malleable.<br />
8. Metal: See <strong>Chapter</strong> 18.<br />
9. Metallic Bond: The chemical bond that binds<br />
metal atoms to other metal atoms in forming metal<br />
substances.<br />
10. Metallic Luster: The shiny appearance of metals.<br />
11. Negative Oxidation State: The state of an atom<br />
which has gained one or more electrons to form an<br />
ionic bond. The precise oxidation state is represented<br />
by a – sign followed by the number of electrons<br />
gained.<br />
12. Nonmetal: See <strong>Chapter</strong> 18.<br />
13. Oxidation Number: A signed number that indicates<br />
how many electrons an atom loses (positive<br />
number) or gains (negative number) by forming a<br />
compound. In ionic bonding the transfer is complete<br />
<strong>and</strong> distinct ions are formed. In covalent<br />
bonding the transfer is only partial <strong>and</strong> distinct ions<br />
are not formed.<br />
14. Positive Oxidation State: The state of an atom<br />
which has lost one or more electrons to form an<br />
ionic bond. The precise oxidation state is represented<br />
by a + sign followed by the number of electrons<br />
lost.<br />
15. Salt: An substance formed from the ionic bond of<br />
a metal with a nonmetal. NaCl is a salt.<br />
16. Thermal Conductivity: A measure of the degree<br />
to which a substance conducts heat. <strong>Metals</strong> have a<br />
high thermal conductivity.<br />
17. Transparency: A characteristic of ionic substances,<br />
such as salts, that readily transmit light.<br />
Opposite to opaqueness.<br />
D. FOCUS QUESTIONS<br />
1. Consider atoms held together in metallic bonds:<br />
a. What happens to the valence electrons when<br />
the bonds are formed?<br />
b. What happens to the energy of the system<br />
when the bonds are formed?<br />
c. What happens to the orbitals when the bonds<br />
are formed?<br />
d. How does this account for the luster of metals?<br />
e. What is the Wave Model of the atom?<br />
f. State the fundamental principle of wave-particle<br />
duality that the Wave Model is based on.<br />
2. Consider the following elements held together in<br />
ionic bonds: (a) sodium, chlorine; (b) magnesium,<br />
chlorine. In each case:<br />
a. Determine the positive or negative oxidation<br />
states of the different atoms.<br />
b. Write the chemical formula for the compound.<br />
c. Write <strong>and</strong> balance the chemical equation for<br />
the reaction.<br />
d. Sketch an energy well for each kind of atom in<br />
the compound. Draw a circle around each electron<br />
soon to be lost in one atom, <strong>and</strong> an empty circle at<br />
the location soon to be filled in the other atom.<br />
e. List the main parts of the Wave Model of the<br />
atom.<br />
f. State the fundamental principle of wave-particle<br />
duality that the Wave Model is based on.<br />
3. Sketch a diagram showing a possible arrangement<br />
of the ions in a salt. Explain what would happen to<br />
this arrangement if shear forces were exerted on the<br />
salt. Why is the salt brittle? Also, using your underst<strong>and</strong>ing<br />
of ionic bonds, explain why table salt dissolved<br />
in water is an ionic conductor. Name <strong>and</strong><br />
state the fundamental principle that explains the<br />
forces that are involved.<br />
E. EXERCISES<br />
<strong>20</strong>.1. When gold <strong>and</strong> silver are mixed, the appearance<br />
is still that of pure gold. How could you distinguish<br />
the alloy from pure gold?<br />
<strong>20</strong>.2 Which of the following pairs of metals is most<br />
likely to form alloys of all compositions?<br />
(a) 56Ba <strong>and</strong> 31 Ga<br />
(b) 50 Sn <strong>and</strong> 82 Pb<br />
(c) 3Li <strong>and</strong> 83 Bi?<br />
<strong>20</strong>.3. Define the following terms: (a) electrical<br />
conductivity, (b) metallic luster, (c) malleability, (d)<br />
thermal conductivity.<br />
<strong>20</strong>.4. Which of the following pairs of metals is<br />
most likely to form alloys of all proportions?<br />
(a) 55Cs <strong>and</strong> 49 In<br />
(b) 78 Pt <strong>and</strong> 79 Au<br />
(c) 82Pb <strong>and</strong> 19 K<br />
<strong>20</strong>.5. In the left half of Figure <strong>20</strong>.4 notice the<br />
prominent diagonal lines of oxidation states. How far is<br />
it vertically from one line to the next? How far horizontally?<br />
<strong>20</strong>.6. How many of the 23 elements listed in the<br />
left half of Figure <strong>20</strong>.4 have only a single oxidation<br />
state?<br />
<strong>20</strong>.7. Write down the primary oxidation state of the<br />
following elements on the basis of their positions in the<br />
Periodic Table:<br />
189
(a) 19K<br />
(b) 10 Ne<br />
(c) 29Cu<br />
(d) 35 Br<br />
(e) 15P<br />
<strong>20</strong>.8. Write down the primary oxidation states of<br />
the following elements <strong>and</strong> check your answers in<br />
Figure <strong>20</strong>.4:<br />
(a) 5B<br />
(b) <strong>20</strong> Ca<br />
(c) 17Cl<br />
(d) 1 H<br />
(e) 18Ar<br />
(f) 28Ni<br />
(g) 24 Cr<br />
<strong>20</strong>.9. Referring to Figure 18.3, select two atoms<br />
that are likely to have negative oxidation states because<br />
they have very high ionization energies. Avoid the<br />
noble gases 2 He, 10 Ne, <strong>and</strong> 18 Ar. Now look these elements<br />
up in Figure 17.7 to see if there is a vacancy for<br />
an electron in a low-lying orbital.<br />
<strong>20</strong>.10. Referring to Figure 18.3, select two atoms<br />
that are likely to have positive oxidation states because<br />
they have low ionization energies. Now look these elements<br />
up in Figure 17.7 to see if the top electrons are<br />
near the top of the well.<br />
<strong>20</strong>.11. Use Figure <strong>20</strong>.4 to write the common oxidation<br />
states of the following elements (e.g., He: 0; <strong>and</strong><br />
C: –2,0,2, <strong>and</strong> 4):<br />
(a) N<br />
(b) P<br />
(c) O<br />
(d) S<br />
(e) Ne<br />
(f) Ar<br />
<strong>20</strong>.12. From Figure 17.6, state which electron Li is<br />
likely to lose to become Li 1+ . Do the same for Be going<br />
to Be 2+ (two electrons).<br />
<strong>20</strong>.13. How many I – ions will be required to neutralize<br />
the charge on an Al 3+ ion?<br />
<strong>20</strong>.14 How many Al 3+ <strong>and</strong> O 2– ions must be combined<br />
to achieve charge neutralization? (Hint: It will<br />
require more than one of each type of ion.)<br />
<strong>20</strong>.15. Write the chemical formulas for the following<br />
combinations:<br />
(a) K + <strong>and</strong> F –<br />
(b) Na + <strong>and</strong> S 2–<br />
(c) Mg 2+ <strong>and</strong> N 3–<br />
<strong>20</strong>.16. Determine the principal oxidation states of<br />
the following elements <strong>and</strong> then write the correct chemical<br />
formulas for the combinations:<br />
(a) 4Be <strong>and</strong> 35 Br<br />
(b) 31 Ga <strong>and</strong> 8 O<br />
<strong>20</strong>.17. Write the names of the following compounds:<br />
(a) BeO<br />
(b) CaCl 2<br />
(c) LiF<br />
(d) Na 2 S<br />
<strong>20</strong>.18. Write the names of the following compounds:<br />
(a) CrO<br />
(b) CrO 3<br />
(c) Cr 2 O 3<br />
(d) NO 2<br />
(e) SO 3<br />
<strong>20</strong>.19. Complete Table <strong>20</strong>.1.<br />
<strong>20</strong>.<strong>20</strong>. Which of the following is an ionic compound?<br />
(a) O 2<br />
(b) NH 3<br />
(c) CO 2<br />
(d) CH 4<br />
(e) MgF 2<br />
is:<br />
<strong>20</strong>.21. The correct formula for potassium sulfide<br />
(a) KS<br />
(b) K 2 S<br />
(c) KS 2<br />
(d) K 3 S<br />
(e) KS 3<br />
<strong>20</strong>.22. Determine the primary oxidation state for<br />
Rb. If two Rb atoms combine with one S atom, what is<br />
the oxidation state of S?<br />
(a) +2<br />
(b) +1<br />
(c) 0<br />
(d) –1<br />
(e) –2<br />
190
Table <strong>20</strong>.1.<br />
NAME<br />
FORMULA<br />
LUSTER OR<br />
COLOR<br />
FORM<br />
CONDUCTIVITY<br />
Sodium fluoride<br />
solid salt<br />
Iron-cobalt alloy<br />
metallic luster<br />
CuCl 2<br />
Calcium oxide<br />
Calcium chloride CaCl 2<br />
Silver-gold alloy<br />
indefinite<br />
Chromium (III) oxide<br />
FeO<br />
Magnesium bromide<br />
Na<br />
191
192