Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3822 A. A. Migdisov, A. E. Williams-Jones, and O. M. Suleimenov<br />
Fig. 5. Published experimental measurements and predicted values<br />
<strong>of</strong> P <strong>AgCl</strong> gas over <strong>chlorargyrite</strong>. The diagram omits some high temper<strong>at</strong>ure<br />
d<strong>at</strong>a (1150 to 1700 K), which appear <strong>in</strong> public<strong>at</strong>ions between<br />
1920 and 1952.<br />
logX <strong>AgCl</strong> H2O n<br />
n 1 logf H2O logK 4 (7)<br />
logX <strong>AgCl</strong> H 2O n<br />
logP<br />
<br />
T<br />
n 1 (8)<br />
Fig. 4. <strong>AgCl</strong> concentr<strong>at</strong>ions <strong>in</strong> the <strong>vapor</strong> versus the pressure <strong>in</strong> the<br />
system. Due to the low partial pressure <strong>of</strong> <strong>AgCl</strong> vap. , the total pressure<br />
<strong>in</strong> the autoclave was effectively th<strong>at</strong> <strong>of</strong> the <strong>w<strong>at</strong>er</strong>. The heavy solid l<strong>in</strong>es<br />
show the silver chloride concentr<strong>at</strong>ion calcul<strong>at</strong>ed assum<strong>in</strong>g no hydr<strong>at</strong>ion<br />
<strong>of</strong> <strong>AgCl</strong>. The calcul<strong>at</strong>ions were done us<strong>in</strong>g the d<strong>at</strong>a on <strong>AgCl</strong> gas<br />
<strong>vapor</strong> pressure over crystall<strong>in</strong>e <strong>chlorargyrite</strong> reported by Tagirov et al.<br />
(1993).<br />
The hydr<strong>at</strong>ion number (n) can be determ<strong>in</strong>ed by the slope <strong>of</strong> the<br />
trend <strong>of</strong> log X <strong>AgCl</strong> (H2 O) n <strong>in</strong> <strong>w<strong>at</strong>er</strong> <strong>vapor</strong>, versus log P H2 O<br />
(Eqs. 7 and 8). This rel<strong>at</strong>ionship is illustr<strong>at</strong>ed <strong>in</strong> Fig. 7.<br />
It is evident from Fig. 7 th<strong>at</strong> the slope is 2 for the temper<strong>at</strong>ures<br />
<strong>in</strong>vestig<strong>at</strong>ed. The rel<strong>at</strong>ively constant slope for all these<br />
temper<strong>at</strong>ures confirms, to a first approxim<strong>at</strong>ion, the assumption<br />
<strong>of</strong> constant stoichiometry <strong>of</strong> the hydr<strong>at</strong>ed complex. However,<br />
those predicted by the more complic<strong>at</strong>ed Pitzer-Palaban model<br />
(Pitzer and Palaban, 1986).<br />
Assum<strong>in</strong>g, th<strong>at</strong> the fugacity <strong>of</strong> <strong>AgCl</strong> (H 2 O) n gas can be<br />
approxim<strong>at</strong>ed as:<br />
f <strong>AgCl</strong> H2On<br />
f H2O X <strong>AgCl</strong> H2O n<br />
(5)<br />
where X <strong>AgCl</strong> (H2 O)n is the mole fraction <strong>of</strong> the hydr<strong>at</strong>ed complex<br />
and f H2 O is the <strong>w<strong>at</strong>er</strong> fugacity, the equilibrium constant <strong>of</strong><br />
reaction (4) can be written as:<br />
logK 4 logX <strong>AgCl</strong> H2O n<br />
logf H2O n logf H2O <br />
logX <strong>AgCl</strong> H2On n 1 logf H2O (6)<br />
To a first, rough approxim<strong>at</strong>ion, it can be assumed th<strong>at</strong> the<br />
fugacity coefficients <strong>of</strong> the components <strong>in</strong> a <strong>vapor</strong> solution do<br />
not differ gre<strong>at</strong>ly from unity, and the total pressure <strong>in</strong> the<br />
system is equal to the pressure <strong>of</strong> <strong>w<strong>at</strong>er</strong>. The equ<strong>at</strong>ions rel<strong>at</strong><strong>in</strong>g<br />
the mole fraction <strong>of</strong> <strong>AgCl</strong> (H 2 O) n gas , partial pressure <strong>of</strong> <strong>w<strong>at</strong>er</strong><br />
and total pressure <strong>of</strong> the system can be obta<strong>in</strong>ed from Eq. 6 as<br />
follows:<br />
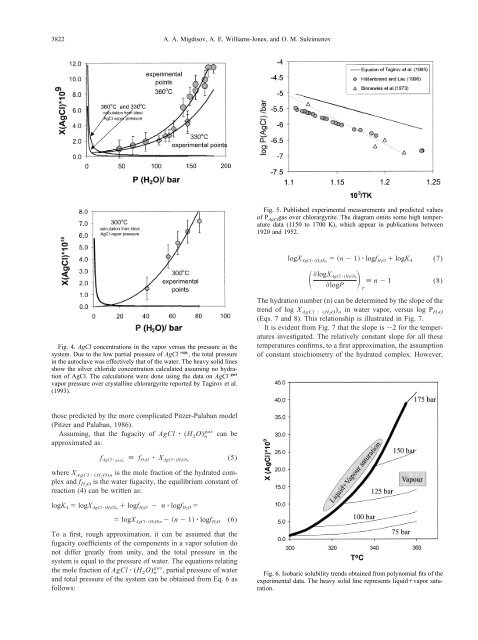
Fig. 6. Isobaric solubility trends obta<strong>in</strong>ed from polynomial fits <strong>of</strong> the<br />
experimental d<strong>at</strong>a. The heavy solid l<strong>in</strong>e represents liquid<strong>vapor</strong> s<strong>at</strong>ur<strong>at</strong>ion.