Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
Solubility of chlorargyrite (AgCl) in water vapor at elevated ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Solubility</strong> <strong>of</strong> <strong>chlorargyrite</strong> <strong>in</strong> <strong>w<strong>at</strong>er</strong> <strong>vapor</strong><br />
3823<br />
therefore conclude th<strong>at</strong> the above complex has a hydr<strong>at</strong>ion<br />
number <strong>of</strong> 3, and we <strong>in</strong>terpret this complex to be<br />
<strong>AgCl</strong> (H 2 O) 3 gas . Identical results were obta<strong>in</strong>ed us<strong>in</strong>g <strong>vapor</strong><br />
densities <strong>in</strong>stead <strong>of</strong> partial pressures <strong>of</strong> <strong>w<strong>at</strong>er</strong>.<br />
<strong>AgCl</strong> cryst 3 H 2 O gas <strong>AgCl</strong> H 2 O 3<br />
gas<br />
(9)<br />
In systems <strong>of</strong> real gases, estim<strong>at</strong>ion <strong>of</strong> <strong>AgCl</strong> solubility is complic<strong>at</strong>ed<br />
by the non-ideal behavior <strong>of</strong> the components and the<br />
<strong>vapor</strong> solution. Fugacity coefficients <strong>of</strong> <strong>w<strong>at</strong>er</strong> <strong>at</strong> the conditions<br />
<strong>of</strong> our experiments differ from 1 and have a pressure dependence.<br />
In order to more accur<strong>at</strong>ely describe a system <strong>in</strong>volv<strong>in</strong>g<br />
real gases, Eq. 8 may by replaced by the follow<strong>in</strong>g equ<strong>at</strong>ion:<br />
logX <strong>AgCl</strong> H 2O n<br />
logP<br />
<br />
T<br />
n 1 1 log H 2O<br />
logP T (10)<br />
The essential difference between the two equ<strong>at</strong>ions is th<strong>at</strong>,<br />
whereas Eq. 8 describes a l<strong>in</strong>ear dependence between log<br />
X <strong>AgCl</strong> (H2 O)n and log P H2 O, Eq. 10 yields a slope, the variability<br />
<strong>of</strong> which depends on the size <strong>of</strong> the term log H 2O<br />
logP<br />
.<br />
T<br />
Interpret<strong>in</strong>g our d<strong>at</strong>a us<strong>in</strong>g this equ<strong>at</strong>ion and the equ<strong>at</strong>ion <strong>of</strong><br />
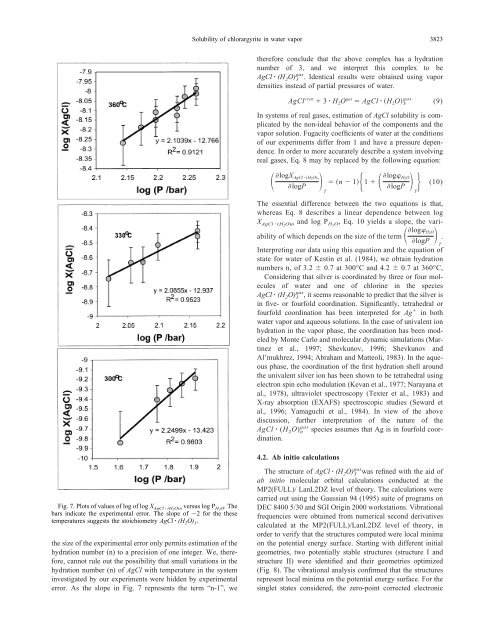
st<strong>at</strong>e for <strong>w<strong>at</strong>er</strong> <strong>of</strong> Kest<strong>in</strong> et al. (1984), we obta<strong>in</strong> hydr<strong>at</strong>ion<br />
numbers n, <strong>of</strong> 3.2 0.7 <strong>at</strong> 300°C and 4.2 0.7 <strong>at</strong> 360°C,<br />
Consider<strong>in</strong>g th<strong>at</strong> silver is coord<strong>in</strong><strong>at</strong>ed by three or four molecules<br />
<strong>of</strong> <strong>w<strong>at</strong>er</strong> and one <strong>of</strong> chlor<strong>in</strong>e <strong>in</strong> the species<br />
<strong>AgCl</strong> (H 2 O) gas 3 , it seems reasonable to predict th<strong>at</strong> the silver is<br />
<strong>in</strong> five- or fourfold coord<strong>in</strong><strong>at</strong>ion. Significantly, tetrahedral or<br />
fourfold coord<strong>in</strong><strong>at</strong>ion has been <strong>in</strong>terpreted for Ag <strong>in</strong> both<br />
<strong>w<strong>at</strong>er</strong> <strong>vapor</strong> and aqueous solutions. In the case <strong>of</strong> univalent ion<br />
hydr<strong>at</strong>ion <strong>in</strong> the <strong>vapor</strong> phase, the coord<strong>in</strong><strong>at</strong>ion has been modeled<br />
by Monte Carlo and molecular dynamic simul<strong>at</strong>ions (Mart<strong>in</strong>ez<br />
et al., 1997; Shevkunov, 1996; Shevkunov and<br />
Al’mukhrez, 1994; Abraham and M<strong>at</strong>teoli, 1983). In the aqueous<br />
phase, the coord<strong>in</strong><strong>at</strong>ion <strong>of</strong> the first hydr<strong>at</strong>ion shell around<br />
the univalent silver ion has been shown to be tetrahedral us<strong>in</strong>g<br />
electron sp<strong>in</strong> echo modul<strong>at</strong>ion (Kevan et al., 1977; Narayana et<br />
al., 1978), ultraviolet spectroscopy (Texter et al., 1983) and<br />
X-ray absorption (EXAFS) spectroscopic studies (Seward et<br />
al., 1996; Yamaguchi et al., 1984). In view <strong>of</strong> the above<br />
discussion, further <strong>in</strong>terpret<strong>at</strong>ion <strong>of</strong> the n<strong>at</strong>ure <strong>of</strong> the<br />
<strong>AgCl</strong> (H 2 O) gas n species assumes th<strong>at</strong> Ag is <strong>in</strong> fourfold coord<strong>in</strong><strong>at</strong>ion.<br />
4.2. Ab <strong>in</strong>itio calcul<strong>at</strong>ions<br />
Fig. 7. Plots <strong>of</strong> values <strong>of</strong> log <strong>of</strong> log X <strong>AgCl</strong> (H2 O)n versus log P H2 O. The<br />
bars <strong>in</strong>dic<strong>at</strong>e the experimental error. The slope <strong>of</strong> 2 for the these<br />
temper<strong>at</strong>ures suggests the stoichiometry <strong>AgCl</strong> (H 2 O) 3 .<br />
the size <strong>of</strong> the experimental error only permits estim<strong>at</strong>ion <strong>of</strong> the<br />
hydr<strong>at</strong>ion number (n) to a precision <strong>of</strong> one <strong>in</strong>teger. We, therefore,<br />
cannot rule out the possibility th<strong>at</strong> small vari<strong>at</strong>ions <strong>in</strong> the<br />
hydr<strong>at</strong>ion number (n) <strong>of</strong> <strong>AgCl</strong> with temper<strong>at</strong>ure <strong>in</strong> the system<br />
<strong>in</strong>vestig<strong>at</strong>ed by our experiments were hidden by experimental<br />
error. As the slope <strong>in</strong> Fig. 7 represents the term “n-1”, we<br />
The structure <strong>of</strong> <strong>AgCl</strong> (H 2 O) 3 gas was ref<strong>in</strong>ed with the aid <strong>of</strong><br />
ab <strong>in</strong>itio molecular orbital calcul<strong>at</strong>ions conducted <strong>at</strong> the<br />
MP2(FULL)/ LanL2DZ level <strong>of</strong> theory. The calcul<strong>at</strong>ions were<br />
carried out us<strong>in</strong>g the Gaussian 94 (1995) suite <strong>of</strong> programs on<br />
DEC 8400 5/30 and SGI Orig<strong>in</strong> 2000 workst<strong>at</strong>ions. Vibr<strong>at</strong>ional<br />
frequencies were obta<strong>in</strong>ed from numerical second deriv<strong>at</strong>ives<br />
calcul<strong>at</strong>ed <strong>at</strong> the MP2(FULL)/LanL2DZ level <strong>of</strong> theory, <strong>in</strong><br />
order to verify th<strong>at</strong> the structures computed were local m<strong>in</strong>ima<br />
on the potential energy surface. Start<strong>in</strong>g with different <strong>in</strong>itial<br />
geometries, two potentially stable structures (structure I and<br />
structure II) were identified and their geometries optimized<br />
(Fig. 8). The vibr<strong>at</strong>ional analysis confirmed th<strong>at</strong> the structures<br />
represent local m<strong>in</strong>ima on the potential energy surface. For the<br />
s<strong>in</strong>glet st<strong>at</strong>es considered, the zero-po<strong>in</strong>t corrected electronic