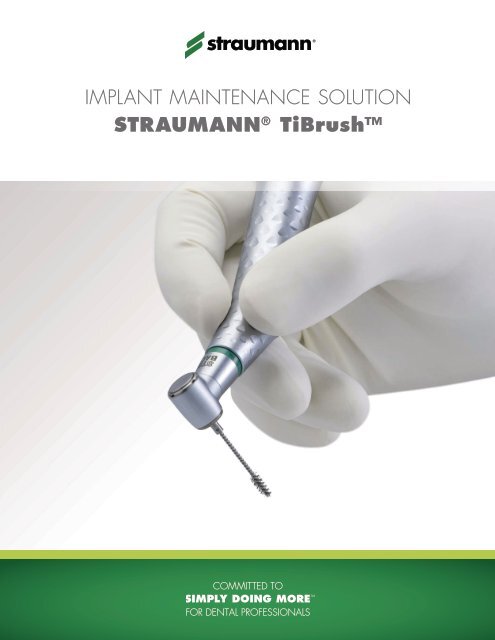
implanT mainTenance soluTion Straumann® tiBrush™
implanT mainTenance soluTion Straumann® tiBrush™
implanT mainTenance soluTion Straumann® tiBrush™
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Implant Maintenance Solution<br />
Straumann ® TiBrush
more effective and efficient at debriding<br />
implant surface than metal cureTTes³<br />
40<br />
Residual plaque measurement (%)<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
TiBrush<br />
Curette<br />
TiBrush removed over 90 % of biofilm within 3 minutes of<br />
use, which is over 3 times more effective than a curette 3 .<br />
Less abrasive on the implant surface²<br />
SEM Investigation of damage to the titanium implant surface shows significantly less<br />
damage caused by TiBrush compared to commonly used metal curettes 1,2<br />
Titanium acting on titanium surface results in less damage to implant surface 2<br />
Untreated surface TiBrush Steel curettes<br />
Pre-op<br />
Initial situation Debridement using Straumann TiBrush* 6 month post-op<br />
Courtesy of: Scott Froum, DDS<br />
*Mechanical debridement was followed by regenerative therapy<br />
1<br />
Compared to steel curettes. 2 Data on file: Benchtest of TiBrush P1.245-001V. 3 Data on file: Benchtest of TiBrush P1.245-008V.
The Straumann ® TiBrush offers clinicians<br />
the following benefits<br />
Gentle on titanium implant surfaces 1,2<br />
More effective and efficient at debriding implant surface 1,3<br />
Significant reduction in treatment time 1,3<br />
Excellent access to implant threads with fine titanium bristles 1<br />
SEM investigation of the uncleaned<br />
implant surface: Implant surface is covered<br />
with a biofilm.<br />
sem investigation of the implant surfacecleaned<br />
with TiBrush.<br />
Courtesy of: Dr. Caspar Wohlfahrt, Institute of Clinical Dentistry, University of Oslo.<br />
Initial situation Debridement using Straumann TiBrush* 3 month post-op<br />
Courtesy of: Robert J. Miller, DMD<br />
*Mechanical debridement was followed by decontamination with PrefGel and tetracycline and Guided Bone Regeneration with<br />
Straumann Allograft and MembraGel
the straumann ® tiBrush<br />
Straumann ® TiBrush (Art. No. 070.005) is a debridement instrument for<br />
dental implants subjected to peri-implantitis.<br />
Straumann ® TiBrush is made of titanium bristles with a stainless steel shaft.<br />
Straumann ® TiBrush is indicated for the open debride ment of titanium<br />
implant surfaces in bone defects caused by peri-implantitis.<br />
Standard dental coupling<br />
Stem<br />
Medical grade stainless steel<br />
Flexible stem for tactile feedback<br />
Brush head<br />
Bristle length optimized to reach into common implant threads<br />
Titanium bristles for less damage to metal implant surface structure 1,2<br />
Brush head size is designed to fit into narrow defects<br />
Gentle on surrounding hard and soft tissue 1<br />
1<br />
Compared to steel curettes.<br />
2<br />
Data on file: Benchtest of TiBrush P1.245-001V.
STEP-by-STEP INSTRUCTIONS<br />
The Straumann ® TiBrush is packaged sterile and must be used<br />
immediately after opening of the blister packaging in an aseptic<br />
surgical environment. The instrument is for single patient use only.<br />
1. Gaining access to the infected implant site<br />
1.1 straumann recommends that the prosthetic supraconstruction<br />
is removed, if possible, prior to use of the Straumann ®<br />
TiBrush.<br />
1.2 use a scalpel to gain access to the infected site. The<br />
Straumann ® TiBrush should only be used in an open flap<br />
surgery.<br />
2. Initial debridement of the implant site<br />
2.1 it is recommended to protect the inner connection of the<br />
implant before debridement with a cover screw or healing<br />
abutment.<br />
2.2 remove excess of granulation tissue around implant with<br />
necessary surgical instruments, taking care not to damage<br />
the implant surface.
3. Debridement of the implant site with the Straumann ®<br />
TiBrush<br />
3.1 visually inspect the package and its contents for damage<br />
before opening the blister. Open the package shortly before<br />
surgery to avoid contamination.<br />
3.2 observing aseptic technique, remove Straumann ® TiBrush<br />
from packaging taking care not to bend the brush. Handle<br />
Straumann ® TiBrush with care to avoid damaging the<br />
bristles prior to treatment.<br />
3.3 attach Straumann ® TiBrush on a surgical handpiece,<br />
which oscillates in a clockwise/counterclockwise direction.<br />
3.4 debride the implant surface using a maximum of 900<br />
oscillations per minute to avoid damaging the integrity<br />
of the implant surface. Use sterile saline solution (NaCl) or<br />
Ringer solution for irrigation and cooling of treatment site.<br />
Tip<br />
The Straumann ® TiBrush can be straightened by hand if it becomes<br />
bent during removal from packaging or during debridement.<br />
Note<br />
It is not recommended to use an oscillating handpiece with an<br />
operating angle greater than ± 30°.
4. Final cleaning of the implant site<br />
4.1 To decontaminate the site and reduce the risk of re-infection,<br />
it is recommended to clean the implant site with a suitable<br />
cleaning agent after debridement.<br />
4.2 rinse extensively with sterile saline solution (Naci).<br />
Note<br />
If the Straumann ® TiBrush is repeatedly used during the same<br />
procedure at multiple sites for the same patient, it is recommended<br />
to immerse the brush in a 3 % hydrogen peroxide solution and<br />
rinse with sterile saline solution between treatment sites.<br />
Practitioners must have appropriate knowledge and instruction<br />
in the handling of the Straumann ® product described herein<br />
(“Straumann Product”) for using the Straumann Product safely<br />
and properly in accordance with these instructions for use. The<br />
Straumann Product must be used in accordance with the<br />
instructions for use provided by the manufacturer. It is the<br />
practitioner’s responsibility to use the device in accordance with<br />
these instructions for use and to determine if the device fits to the<br />
individual patient situation. Pictures show generic implant.
www.straumannusa.com<br />
International Headquarters<br />
Institut Straumann AG<br />
Peter Merian-Weg 12<br />
ch-4002 Basel, Switzerland<br />
Phone +41 (0)61 965 11 11<br />
Fax +41 (0)61 965 11 01<br />
© Straumann USA, LLC 2013. All rights reserved.<br />
Straumann USA<br />
Straumann USA, llc<br />
60 Minuteman Road<br />
Andover, MA 01810<br />
Phone 800/448 8168<br />
978/747 2500<br />
Fax 978/747 2490<br />
www.straumannusa.com<br />
Straumann Canada<br />
Straumann Canada Limited<br />
3115 Harvester Road, Suite 100<br />
Burlington, ON L7N 3N8<br />
Phone 800/363 4024<br />
905/319 2900<br />
Fax 905/319 2911<br />
www.straumann.ca<br />
Straumann ® and/or other trademarks and logos from Straumann ® that are mentioned herein are the trademarks or registered trademarks of Straumann Holding<br />
AG and/or its affiliates. All rights reserved.<br />
1/13 USLIT 434